| Feb 08, 2011 |
First images of proteins and viruses caught with an X-ray laser
|
|
(Nanowerk News) It has been a dream of researchers for over a decade: image biological materials at high resolution using incredibly intense X-ray laser pulses. Calculations had long predicted that these blasts of X-rays would allow exquisite measurements of the molecular structure of biological objects, from samples too small to be studied by conventional methods. In the usual methods, X-ray images of materials are blurred out because the X-rays degrade the structure. Surprisingly, the proposed way to overcome this "radiation damage" is to use ultra-short pulses a billion times brighter than conventional sources. The pulses are so fast that the image is formed before the effects of the damage are felt.
|
|
Now, an international collaboration, led by Professor Henry Chapman of the Center for Free-Electron Laser Science (CFEL) at DESY in Hamburg, Germany, has proven this principle at the Linac Coherent Light Source (LCLS, at SLAC National Accelerator Laboratory in California, USA) by forming images of the Photosystem I protein complex and particles of the Mimivirus. The results, published in two papers in Nature, open a way for obtaining the molecular structures of proteins and viruses without the requirement of high-quality crystals. By using single virus particles or nanocrystals of proteins too small to see in an optical microscope, it is possible to streamline structural studies and avoid the years of laborious crystallization trials that are often needed to determine a detailed structure.
|
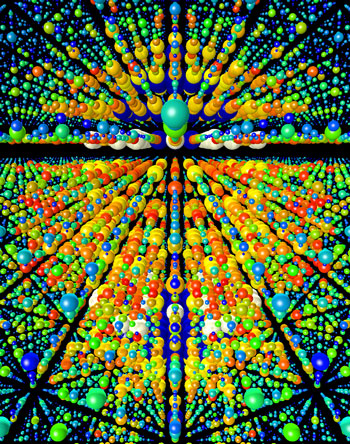 |
| Three-dimensional rendering of X-ray diffraction data obtained from over 15 000 single nanocrystal diffraction snapshots recorded at the LCLS. Each nanocrystal was destroyed by the intense X-ray pulse, but not before information about its structure was revealed. Nanocrystals of photosystem I were produced by Petra Fromme of Arizona State University.
|
|
"These achievements are a culmination of many years of effort that began with prototype experiments at DESY´s FLASH facility, the free-electron laser in Hamburg," said Professor Henry Chapman. "They were only possible with a large multidisciplinary effort involving physicists, biologists, specialists in optics, plasmas, and detectors. Although the X-ray patterns we obtained from single nanocrystals were exactly as we predicted, just like in text-books, we were surprised and thrilled as to how well the experiments worked."
|
|
The 3D structures of proteins and viruses are conventionally recorded by a method called X-ray crystallography. An X-ray beam shining on the crystal scatters into an arrangement of spots called a diffraction pattern. The measurement of the intensities of these spots as the crystal is rotated through the beam can be interpreted to give the 3D image of the protein or virus. However, the ionizing X-rays destroy the very object under investigation, so large exquisite crystals are needed to get strong patterns before radiation damage sets in.
|
|
The method often fails because defect-free crystals cannot be grown, despite years of effort. The LCLS X-ray laser produces pulses of X-rays that are a billion times brighter than the conventional synchrotron sources used to date. A single pulse is so intense that any sample placed in its path is vaporized into a plasma that is hotter than the sun. But this destruction only happens after the brief X-ray pulse passes through the object. At only 100 femtoseconds long (1 femtosecond = 10-15 seconds) the X-ray pulse is faster than the time it takes the atoms to move and commence the explosion. The X-ray pattern carries the information about the undamaged object. The pulses are so intense that tiny nanocrystals, or even single virus particles, give strong enough patterns for interpretation, in agreement to calculations made over 10 years ago by Professor Janos Hajdu (Uppsala University) and colleagues.
|
|
"These experiments are a breakthrough on our roadmap to single object X-ray diffraction," said Professor Helmut Dosch, Chairman of the DESY Board of Directors. "We see a glimpse of the world of tomorrow, in which X-ray lasers finally get to grips with non-crystalline matter."
|
|
The studies in Nature demonstrate this concept of "diffraction before destruction" in spectacular style. Each X-ray pulse gives a single view of the object and the formation of a 3D image requires many snapshots of randomly oriented but identical particles. The experiments made use of the CAMP chamber and pnCCD detector system, designed and built by the Max-Planck Advanced Study Group at CFEL, a cooperation of eight Max-Planck Institutes, as well as the recent invention of a gas-focused liquid jet at Arizona State University (ASU). The target objects are fed into the X-ray beam in an aerosol beam (developed at LLNL, Stanford PULSE Institute for Ultrafast Energy Science, and Uppsala University) or a liquid jet (developed at ASU) and 1,800 individual patterns are collected every minute. By the time the next pulse arrives, at a rate of 30 per second, a new particle is delivered to replace the vaporized one before it. The high-speed pnCCD X-ray detectors from the Max Planck Semiconductor laboratory and Max-Planck Advanced Study Group recorded and digitized millions of patterns over the course of several days. Many terabytes of data were collected—the data rate exceeds that of particle physics experiments at the Large Hadron Collider (LHC). These data were analyzed on computer clusters at SLAC and DESY, using parallel programs that determine the orientation of the crystal to build up the 3D diffraction pattern that was a composite of tens of thousands of the best patterns. This composite could then be processed as if collected from a single perfect crystal.
|
|
Photosystem I is is one of Nature's most important biological machines: the complex converts sunlight to energy during photosynthesis. The Photosystem I nanocrystals were an ideal sample for the proof-of-principle experiments. The crystals, ranging in size from about 100 nanometers to 2 micrometers were produced by Professor Petra Fromme of Arizona State University. They are a class of membrane-bound proteins, which are extremely fragile and are the hardest to crystallize. Less than 300 membrane-bound protein structures have been determined to date (compared with many tens of thousands of soluble proteins), despite their importance as drug targets. The structure of the Photosystem I complex had been previously determined using large crystals that took
13 years of effort to find crystallization conditions. This allowed a quantitative comparison that proved the accuracy of the method using the much more easily produced nanocrystals, and showed that "diffraction before destruction" is viable even with extremely fragile objects.
|
|
The ultimate application of the "diffraction before destruction" technique is to record patterns from single molecules, without the need for any crystallization at all. This goal requires further development to focus the X-ray pulses to smaller spots, giving even higher intensities. This didn't stop the ambitions of the collaboration to carry out yet another spectacular proof of principle experiment on imaging single copies of the giant Mimivirus, the world's largest known virus, which infects amoebas. The experiments show the feasibility of eschewing crystallinity altogether, which gives rise to continuous diffraction patterns instead of ones consisting of spots. Thousand of patterns of single viruses were recorded, and single images were reconstructed. This new way of imaging avoids having to freeze, slice, or chemically label the structure, and could be extended to whole living cells.
|
|
The collaboration conducting the pioneering experiment was made up of 85 members from CFEL at DESY, Arizona State University, the Max Planck Institute for Medical Research and the Max Planck advanced study group at CFEL, Uppsala University, SLAC, Stanford PULSE Institute, Lawrence Livermore National Laboratory, and Lawrence Berkeley National Laboratory. The techniques demonstrated by the collaboration will continue to be developed to higher resolution. The LCLS now provides wavelengths of X-rays short enough to resolve interatomic spacings. Like flash photography, the short X-ray pulses mean that fast molecular processes in proteins can be frozen in time, so reactions can be filmed and the function of biological macromolecules determined. The research shows the enormous potential of X-ray free-electron lasers, and spurs the development of new facilities such as the European XFEL in Hamburg.
|

