| Dec 05, 2011 |
Record high two million gold nanoparticles inserted into a single cancer cell
|
|
(Nanowerk News) By changing the organic molecule that coats gold nanorods, scientists at Rice University in Houston have increased the number of gold nanorods inserted into a single, living cell to over 2 million. The previous record was 150,000. This achievement could greatly improve photothermal therapy for cancer, which uses near-IR light to heat nanorods inside cancer cells to destroy them.
|
|
"It should be obvious that the more particles you have inside the cell the more efficient this treatment will be," says Eugene Zubarev, the lead scientist on the project. "If you only have a limited number of particles the amount of heat generated inside may not be enough to kill the cell. So the more the better."
|
|
As reported in Angewandte Chemie ("Quantitative Replacement of Cetyl Trimethylammonium Bromide by Cationic Thiol Ligands on the Surface of Gold Nanorods and Their Extremely Large Uptake by Cancer Cells"), Zubarev and co-workers achieved this large concentration of nanorods inside the cell by changing the ligand attached to the gold nanorods from the commonly-used cetyl trimethylammonium bromide (CTAB) to (16-mercaptohexadecyl) trimethyl ammonium bromide (MTAB). MTAB has thiol (S-H) groups at the ends of its chain instead of the CH3 groups that terminate CTAB. While the CH3 groups are basically inert, "once you have a thiol group in your organic molecule, that thiol will find the surface of gold and essentially bind to gold covalently," Zubarev says. "So it is really a switch from non-covalent to covalent binding, which makes an intractable system tractable."
|
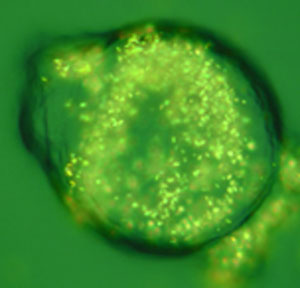 |
| Optical microscopy image of a cancer cell treated with gold nanorods. (Image: Eugene Zubarev, Rice University)
|
|
This switch to covalent bonding means the MTAB remains attached to the gold nanorod inside the cell at all times. With CTAB there is always dynamic exchange, Zubarev says, with some fraction of CTAB molecules free in solution inside the cell, not attached to the nanorods. This causes CTAB to be cytotoxic. MTAB does not go into solution, so MTAB-coated nanorods proved to be non- toxic to the cells.
|
|
"It's always been a mystery when it comes to the application of nanorods—what is causing the toxicity?" Zubarev says. "Is it the free ligand which is constantly leaching out into solution, or is it the nanostructures themselves? Many people say CTAB-coated rods themselves are cytotoxic. But I think this particular study has shown that if you ensure that there is no free ligand in solution then cytotoxicity will disappear."
|
|
The mechanism responsible for the great increase in the number of nanorods in a single cell is not understood, but Zubarev and his team have a working hypothesis. They counted approximately 5,000 cationic MTAB ligands coating the surface of each nanorod, giving each a charge of +5,000. The membranes of cells are negatively charged, resulting in an electrostatic interaction between the nanorods and the cells. "You have a very high density of positive charge considering that the size of a nanorod is very small. In my opinion that's the most important thing—the really high charge density, and the multi-ligand agent, so it can cover a relatively large area on the surface of a cell, and interact favorably with it," Zubarev says, allowing that his hypothesis could change as more data is obtained. "The higher the charge density on your particle the more strongly it can initially attach to the surface of the cell and get inside."
|
|
Future work to test this hypothesis will involve substituting polymeric macromolecules with lower cationic charges for MTAB ligands. Comparing the uptake of nanorods into cells in these two systems could reveal just how important charge density is.
|

