| Jan 02, 2013 |
Bacterial suicide: assembly of a lethal molecular machine
|
|
(Nanowerk News) How can bacteria protect themselves from lethal infection by viral parasites? One extreme way is for individual cells to commit suicide when infected, thereby preventing or limiting viral replication and protecting the rest of the bacterial population from subsequent infections. In the bacterial plant pathogen, Pectobacterium atrosepticum, this "altruistic suicide" can be mediated by an RNA-protein complex called ToxIN. The suicide complex is not induced but exists as a constitutively produced system that is held in a suppressed, inert form in the cell until viral infection triggers the release of the protein toxin (ToxN) from its RNA antitoxin (ToxI) partner. This toxin causes the death of both the bacterium and the infecting virus.
|
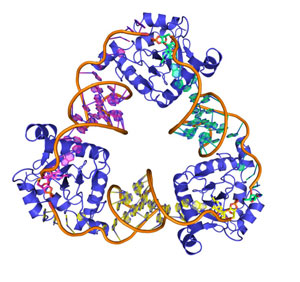 |
| A computer-generated image of the ToxIN complex from Bacillus thuringiensis. (Image: Francesca Short)
|
|
Such a strategy has high risks, because the bacterium must have the means of suicide to hand at all times. The success of this antiviral system therefore depends heavily on maintaining a very strong inhibition or suppression of the toxin by its RNA antitoxin, to ensure that the host cell is not damaged in the absence of invading viruses. Small RNAs have multiple essential roles in bacteria, but examples of naturally occurring RNA molecules that act as direct protein inhibitors are rare. A study by BBSRC-funded researchers at the University of Cambridge , published in Proceedings of the National Academy of Sciences (PNAS) explores the powerful ToxN-inhibiting activity of the ToxI RNA.
|
|
It shows that the ToxI RNA "neutralizes" its toxin partner through the self-assembly of a beautiful, triangular ToxI-ToxN macromolecular complex, previously observed by earlier BBSRC-funded crystallographic studies (Blower et al, 2011, Nature Structural and Molecular Biology, 18, 185-190). The assembly of the inhibited complex is driven entirely by the sequence of the ToxI RNA, and its interactions with ToxN. The study shows that ToxI RNAs are highly selective inhibitors, each active against only their own specific toxin partner. The structure of a second ToxI-ToxN complex, encoded by a plasmid of the bacterium, Bacillus thuringiensis, reveals that this selectivity is a consequence of subtle, complementary structural variations in both RNA and protein; the precise molecular recognition needed to form an inactive complex cannot occur between mismatched partners.
|
|
In addition, the work shows that ToxIN systems promote their own maintenance on plasmids as selfish DNA that likely increases their spread, and retention, in bacterial populations.
|
|
Professor George Salmond, deputy head at Cambridge University's Department of Biochemistry, said: "The results present a picture of ToxIN as an addictive, self-assembling - and potentially lethal - molecular machine, which can drive remarkable adaptive advantages in populations of bacterial hosts, including those under threat from lethal viral predation."
|
|
Discovering the mode of assembly, and molecular activation, of lethal ToxIN complexes might be exploited in the longer term to enable the discovery and development of new small molecule antibacterials.
|

