| Sep 23, 2013 |
Freeze! A protein group affecting lipid dynamics at cell membranes discovered
|
|
(Nanowerk News) Eukaryotic cells are compartmentalized by membranes, whose shape and dynamics are precisely regulated to maintain their correct functions. Consequently, many cellular processes such as endocytosis, migration and morphogenesis rely on proteins that bind directly to membranes and sculpt them into desired shapes.
|
|
BAR domain proteins are among the central membrane-sculpting proteins in all eukaryote cells. Studies by Pekka Lappalainen laboratory at Institute of Biotechnology, University of Helsinki, Finland, now reveal that BAR domain proteins not only bend membranes, but also generate extremely stable lipid microdomains by inhibiting the lateral diffusion of certain lipids nearly completely.
|
|
In a new study published in Cell Reports ("Membrane-Sculpting BAR Domains Generate Stable Lipid Microdomains"), Hongxia Zhao working in the Lappalainen laboratory discovered that all BAR domain proteins induce strong clustering of phosphoinositides, which are important lipids involved in regulating protein functions and cellular signalling.
|
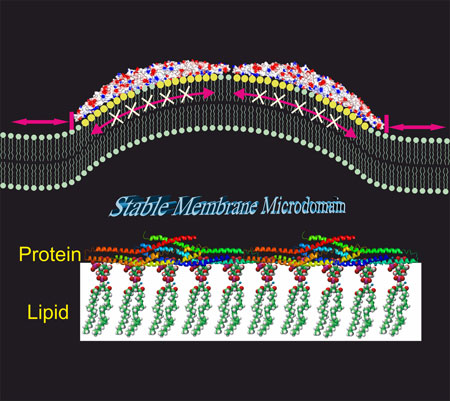 |
| BAR proteins can create stable lipid microdomains at cell membranes. (Image: Hongxia Zhao)
|
|
Her studies also revealed that BAR domains assemble into extremely stable scaffolds on the membrane. Surprisingly, mobility of phosphoinositides was nearly completely frozen in these BAR domain induced lipid platforms. These extremely stable protein-lipid scaffolds may contribute to diverse cellular processes by generating lipid phase boundaries at the tips of the BAR domain scaffolds. Furthermore, the membrane microdomains induced by BAR domains are expected to function as diffusion barriers, which may for example trap membrane-associated receptor and cargo molecules at the endocytic bud.
|
|
Thus, distribution and mobility of specific lipid species at the plasma membrane appears to precisely regulated by membrane-associated proteins. Hongxia Zhao is now continuing studies on regulation of lipid dynamics as an Academy Research Fellow at University of Helsinki.
|

