| Jul 26, 2015 |
Bracing up DNA
|
|
(Nanowerk News) The iconic image of DNA is that of a double helix; but when the DNA is about to be “read out,” replicated or repaired, the two strands of the helix come apart. A single strand is more accessible than the double helix when it comes to reading out genetic information, but it is also less stable and more vulnerable to environmental damage. Weizmann Institute researchers have now revealed the mechanism of action of the “back brace” proteins that stabilize single strands, protect them and prevent them from folding back into a double-helix structure that can inhibit subsequent DNA processing.
|
|
These proteins – known as specialized single-stranded DNA-binding proteins – exist in numerous life forms, including humans. In the new study, reported in the Proceedings of the National Academy of Sciences ("Molecular determinants of the interactions between proteins and ssDNA"), Prof. Yaakov (Koby) Levy and postdoctoral fellow Dr. Garima Mishra of the Weizmann Institute's Structural Biology Department have built a computational model that helps reveal how the “back brace” proteins operate.
|
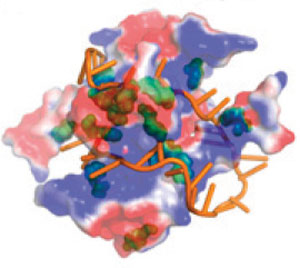 |
| Single-stranded DNA (orange) interacts with the negatively (red) and positively (blue) charged parts of the protein molecule, as well as with its amino acid structures called aromatic groups (green).
|
|
As the helix opens, various proteins slide along the double-stranded DNA searching for their unique binding spot, and the “back brace” proteins, too, slide on the single-stranded DNA. Usually interactions between sliding proteins and DNA are based on differences in electrical charges: positive for the proteins and negative for the DNA. The new Weizmann Institute model has revealed that in the case of the “back brace” proteins, the situation is more complex. These proteins also interact with the single DNA strand chemically, via amino acids possessing structures called aromatic groups. Through these two types of interaction, the “back brace” proteins and the single strand wrap around each other until they form a stable complex.
|
|
With the help of their model, the Weizmann researchers correctly predicted the precise configuration of such complexes: Their predictions were validated by comparison with actual structures that had earlier been solved experimentally for crystallized protein-DNA complexes.
|
|
The theoretical backing offered by the Weizmann study can facilitate new experimental investigations. In particular, says Levy, scientists can now investigate whether previous discoveries made about interactions between proteins and double-helix DNA also apply to protein interactions with single DNA strands. For example, in the past few years he and his team had helped to resolve the speed-stability paradox: how proteins sliding along the double helix manage to swiftly find appropriate spots for binding, yet at the same time to create bonds that are sufficiently strong to support molecular interactions. Using computational analysis, the team had identified geometrical and energetic parameters that enable the proteins to bind with the DNA at the optimal speed and strength.
|
|
By shedding new light on how the DNA functions at the molecular level, this study advances the understanding of biological processes that form the very basis of life and may lead to improved biotechnological applications based on protein-DNA interactions.
|

