| Jun 16, 2015 |
Key to quick battery charging time
|
|
(Nanowerk News) University of Tokyo researchers have discovered the structure and transport properties of the "intermediate state" in lithium-ion batteries - key to understanding the mechanisms of charge and discharge in rechargeable batteries. These findings ("Superstructure in the Metastable Intermediate-Phase Li2/3FePO4 Accelerating the Lithium Battery Cathode Reaction") may help accelerate battery reaction speed and significantly shorten battery charging time.
|
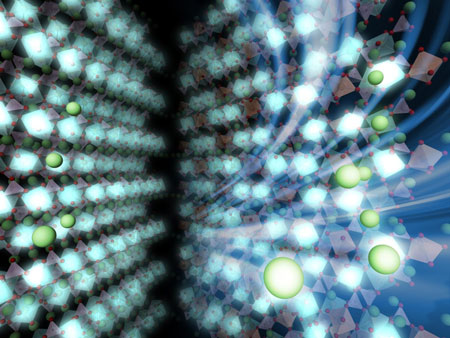 |
| The left side of the image illustrates the discharged state with limited movement of electrons and lithium ions. The right side shows the intermediate state. The white area is densely packed with electrons whereas the brown area is loosely packed, forming a striped pattern. Lithium ions distribute themselves so as not to disturb the striped pattern. In the intermediate state, both electrons and lithium ions can move much faster, leading to a faster battery charging time. (Image: Atsuo Yamada)
|
|
Although there is strong demand to minimize battery-charging time, the mechanisms of battery charge and discharge reactions have yet to be fully understood. While the existence of an "intermediate state" that accelerates battery charge and discharge reactions has been suggested, there was no firm experimental evidence to support its existence and previous research had suggested that the short lifetime of the intermediate state would render a systematic investigation of its properties impossible.
|
|
Now, Professor Atsuo Yamada's research group at the University of Tokyo Graduate School of Engineering have developed a novel technique to stabilize the intermediate state. The group found a striped pattern of layers of densely and loosely packed electrons. Lithium ions distribute themselves so as not to disturb this striped pattern. In addition, the intermediate state showed high lithium/electron conductivity compared to the charged or discharged state. That is, both lithium ions and electrons could move faster in the intermediate state, contributing significantly to accelerating lithium-ion battery charge and discharge reactions.
|
|
The findings were contrary to expectations. "The intermediate state showed a long lifetime, once we were able to optimize the synthesis conditions. We were also successful in stabilizing the intermediate state with almost 100% purity," says Yamada. "It was surprising to find that conductivity was hugely enhanced in the intermediate state. We hope to develop rechargeable batteries with quick charging time by applying our findings to the design of materials and conditions that are optimum for creating a stable intermediate state."
|

