| Jul 17, 2012 |
Weak light can now be used for applications as well - researchers harvest infrared light
|
|
(Nanowerk News) Chemists and materials scientists from the University of Groningen and the FOM Foundation have found a way of ‘harvesting‘ infrared light more efficiently. For this they use special molecules, which act as light antennae to capture the energy from weak infrared light. The antennae transmit the energy to the nanoparticles they are attached to. These particles subsequently convert two weak captured photons into a single strong, energy-rich photon, a process termed upconversion. The new antennae molecules amplify this process 3300 times, which represents a considerable improvement for solar cells or medical imaging techniques, for example. The research was published on 15 July 2012 on the website of the journal Nature Photonics ("Broadband dye-sensitized upconversion of near-infrared light").
|
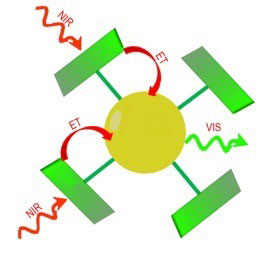 |
| Figure 1. Diagram of the nanocrystal with infrared-absorbing antennae. NIR = near infrared, ET = energy transfer, VIS = emission of visible light.
|
|
Infrared light has too little energy to release electrons in solar cells, for example. This relatively weak light is therefore lost. One possible way of still being able to use infrared light is ‘upconversion’: ‘adding up’ the energy of two weak photons to produce a single stronger photon (visible light).
|
|
Factor of 3300 increase
|
|
"There are inorganic materials made from rare earth metals that can facilitate this upconversion process," explains University of Groningen Professor of Organic Chemistry Kees Hummelen, who leads the FOM-focus group 'Next generation organic photovoltaics'. "However these materials absorb very few infrared photons. We have therefore attached organic molecules to them as antennae that can capture these photons and transmit the energy to the upconversion material. With this, the entire process of infrared absorption, upconversion and the emission of visible light is being increased by a factor of 3300."
|
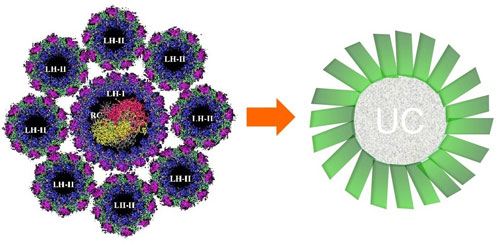 |
| Figure 2. Inspiration from nature. Left a natural photosynthesis system with light-harvesting molecules (LH) and a reactive centre (RC), right a schematic representation of the nanocrystal that realises the upconversion (UC) with the attached antennae in green.
|
|
Harvest
|
|
Hummelen’s group is trying to further increase the harvest of infrared photons. "Even with our antennae we still only capture a limited amount of the infrared light. And even better yield can be obtained," predicts Hummelen. However the process of upconversion inside the nanocrystal is still rather inefficient. "Two photons must come together in the material within a short space of time. In practice the efficiency of this process is still very low. However the harvest is already much better, so step 1 has been achieved!" says Hummelen. Therefore, the work of the researchers in Groningen is mainly a proof that infrared photons can be harvested by means of upconversion.
|
|
Solar cells
|
|
The most obvious application is in solar cells, as about half of all the solar energy reaching the Earth's surface consists of infrared light. "A German group is going to incorporate our nanocrystals with antennae in solar cells to test these in practice," says Hummelen, who since last year has led a FOM focus group researching the new generation of organic solar cells. By capturing more infrared light, solar cells will be able to pass the so-called Shockley-Queisser efficiency limit. For solar cells that consist of one color that limit is 32 percent.
|
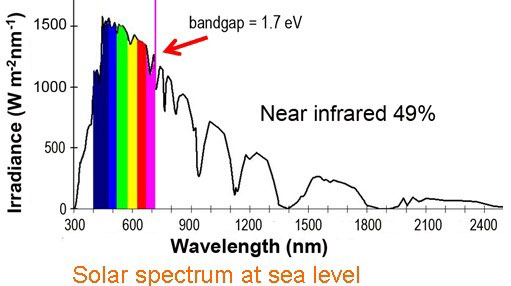 |
| Figure 3. Spectrum of sunlight at sea level. About 49% of the radiation is in the infrared or near infrared.
|
|
Medical images
|
|
The system for upconversion of infrared light also has other applications, including medical imaging techniques. "Infrared light penetrates further into biological tissues than visible light. If you allow compounds that carry out upconversion to bind to specific cells in tissues then you can make images using infrared light," explains Hummelen.
|
|
Worlds combined
|
|
In the FOM focus group at the University of Groningen researchers combine organic and inorganic chemistry. "These worlds are usually still quite distinct," says Hummelen. "We have brought them together, with this success as the result." They modified an organic dye that can absorb infrared light so that this could be attached as an antenna to a lanthanide nanocrystal. About 60 of these antennae are attached to a single crystal. "If you try to place more on the crystal then they disrupt each other." The antennae capture the infrared energy and transmit it to the nanocrystal, which subsequently uses it to produce energy-richer photons that have enough energy to release an electron in solar cells, for example. "Our inspiration partly came from the photosynthesis complex of plants," explains Hummelen. “There you have a ring of light-absorbing molecules around an active centre."
|



