| Oct 24, 2012 |
Nanomaterials: Bringing crystals into line
|
|
(Nanowerk News) The unique magnetic properties of cobalt phosphide nanowires stand them in good stead as future components of high-performance devices. Unlike bulk materials, these ultrasmall elongated crystals consist of single-domain structures that account for their superparamagnetism — a temperature-induced magnetism that arises in a magnetic field. To maintain and fully exploit this behavior, scientists must generate materials composed of precisely positioned and oriented building blocks. Such superstructures are now available, thanks to the development of a method that uses temperature changes to align individual nanowires. Ming-Yong Han from the A*STAR Institute of Materials Research and Engineering, Sinapore, led the research (see paper in Advanced Materials: "Temperature and Chemical Bonding-Directed Self-Assembly of Cobalt Phosphide Nanowires in Reaction Solutions into Vertical and Horizontal Alignments").
|
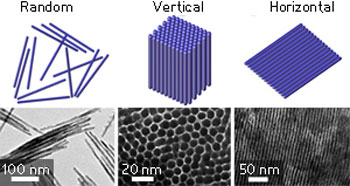 |
| Schematic representations (top) and transmission electron microscopy images (bottom) of randomly oriented and vertically and horizontally aligned cobalt phosphide nanowires. (© Wiley-VCH Verlag)
|
|
Current nanocrystal self-assembly approaches involve depositing a crystal suspension on a solid surface, and then slowly evaporating the solvent. Theoretically, the evaporation enhances the relatively weak attraction forces that exist between the nanocrystals, forcing them to align. However, high degrees of alignment of anisotropic structures — those exhibiting direction-dependent physical properties — remain difficult to achieve.
|
|
“We took a distinct pathway from the slow evaporation approach,” says Han. His team’s strategy followed similar principles to those used in chemical synthesis. First, they reacted a cobalt derivative with the phosphide precursor trioctylphosphine (TOP) at high temperature. This produced TOP-coated nanowires. Next, they stored the solution in which the nanowires formed at various temperatures. These storage, or ‘aging’, temperatures produced larger, well-defined superstructures with different alignments.
|
|
Washing the nanowires without the latter step resulted in random arrangements or small assemblies (see image). After cooling and aging the reaction mixture at room temperature for two hours, the team observed superstructures composed of nearly one million vertically standing nanowires. In this arrangement, each nanowire was surrounded by six others in a honeycomb pattern. When cooled to room temperature and then refrigerated, the reaction mixture produced extended sheets of nanowires aligned side-by-side horizontally.
|
|
The superstructures resisted any high temperature, ultrasound, or organic solvent treatment, indicative of strong cohesive forces between the nanowires. Further investigations revealed that, during the self-assembly, the TOP molecules continually adsorbed and desorbed from the nanowires, bringing them in close contact. This caused irreversible chemical bonds to form between the nanocrystals, facilitating and enhancing their alignment.
|
|
The team is currently testing the performance of the superstructures against that of the randomly oriented nanowires to explore their potential use as sensors or electrical components called inductors. “We are also trying to extend this methodology to self-assemble other systems, with a hope to establish a more universal method for aligning anisotropic nanocrystals,” adds Han.
|

