Definition:: Graphdiyne is a two-dimensional carbon allotrope, similar to graphene, but with a distinct atomic structure comprising sp and sp2 hybridized carbon atoms. This unique structure endows it with a range of physical and chemical properties different from those of graphene, making it a material of significant interest in nanotechnology and materials science.
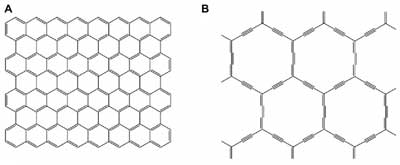
Structures of A) graphene and B) α-graphyne. (Reprinted with permission by The American Physical Society)
Difference Between Graphdiyne and Graphene
While both graphdiyne and graphene are composed of carbon atoms, their structural differences lead to distinct properties and potential applications. Graphene consists of a single layer of carbon atoms arranged in a hexagonal lattice, all sp2 hybridized, giving it remarkable strength, flexibility, and electrical conductivity.
Related Carbon-Based Nanomaterials
More generally, and looking beyond graphene, Graphdiyne belongs to a fascinating family of two-dimensional materials composed purely of carbon atoms. Here are some closely related materials with intriguing properties of their own:
- Graphyne: Graphyne shares structural similarities with graphdiyne, featuring both sp and sp2 hybridized carbon atoms. However, it differs in the arrangement and number of acetylenic linkages within its structure. This variation creates the potential for different electronic and chemical properties.
- Graphone: Graphone is a partially hydrogenated form of graphene. It can also be considered a precursor to graphdiyne, as removing hydrogen atoms from graphone can lead to the formation of graphdiyne's triple bond structure.
- Graphane: Graphane is fully hydrogenated graphene, where each carbon atom is bonded to a hydrogen atom. This results in a vastly different material from graphene with insulating properties, rather than conductive ones.
Research into these related
nanomaterials is ongoing, with the potential to unlock new applications alongside graphdiyne in fields like electronics, energy storage, and catalysis.
Graphdiyne, on the other hand, incorporates both sp and sp2 hybridized carbon atoms. This configuration results in a diacetylenic linkage (a repeating unit of two triple bonds separated by a single bond) within its hexagonal lattice, offering a wider bandgap and potentially making it more suitable for semiconducting applications than graphene.
Properties and Applications
- Electronic Properties: Graphdiyne's unique atomic structure gives it a bandgap, which is crucial for its application in semiconductors, photovoltaics, and electronic devices.
- Chemical Reactivity: The presence of triple bonds in graphdiyne's structure makes it more chemically reactive than graphene. This property is advantageous for sensor applications and catalysis.
- Porosity: Graphdiyne has a highly porous structure, making it suitable for gas storage and separation applications, as well as in energy storage devices like batteries and supercapacitors.
Key Features
Graphdiyne's bandgap can be tuned through chemical modifications, a property not inherent in graphene. This tunability, along with its porosity and chemical reactivity, broadens the scope of its applications, potentially surpassing those of graphene in certain areas.
Challenges and Research Directions
Despite its promising properties, the synthesis of graphdiyne is challenging, limiting its large-scale production and application. Research is ongoing to develop efficient and scalable synthesis methods, as well as to explore and expand the applications of graphdiyne in nanotechnology, electronics, and energy storage.
Further Reading