Nanoshells: Enhancing Optical Imaging and Photothermal Therapy
What are Nanoshells?
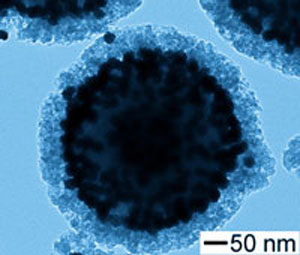
Nanoshells are a class of nanoparticles consisting of a dielectric core surrounded by a thin metallic shell, typically made of gold or silver. The core material is usually silica or a polymer, while the shell thickness ranges from a few to tens of nanometers. By tuning the core-shell ratio and the overall particle size, nanoshells can be engineered to strongly absorb or scatter light at specific wavelengths, making them valuable tools for biomedical imaging and therapy applications.
Optical Properties of Nanoshells
The optical properties of nanoshells arise from the interaction between the core and shell materials, as well as the particle size and shape. When light strikes a nanoshell, it induces collective oscillations of the conduction electrons in the metallic shell, known as localized surface plasmon resonance (LSPR). The LSPR wavelength depends on the core-shell ratio and the dielectric properties of the surrounding medium.
Tuning the LSPR Wavelength
One of the key advantages of nanoshells is the ability to tune their LSPR wavelength by adjusting the core-shell ratio. For example, increasing the shell thickness relative to the core size shifts the LSPR to shorter wavelengths, while decreasing the shell thickness shifts it to longer wavelengths. This tunability allows researchers to design nanoshells that strongly absorb or scatter light in the near-infrared (NIR) region, which is particularly useful for biomedical applications due to the deep tissue penetration of NIR light.
Absorption and Scattering Cross-Sections
Nanoshells exhibit large absorption and scattering cross-sections at their LSPR wavelength, which can be several orders of magnitude higher than those of conventional dyes or fluorescent probes. The relative contributions of absorption and scattering to the total extinction cross-section depend on the nanoshell size and core-shell ratio. Smaller nanoshells (<50 nm) tend to have higher absorption cross-sections, while larger nanoshells (>100 nm) have higher scattering cross-sections.
Nanoshell Synthesis Methods
Several methods have been developed for the synthesis of nanoshells with controlled size, shape, and composition. The choice of the synthesis method depends on the desired material properties, scalability, and application requirements. Some of the common nanoshell synthesis methods include:
Template-Assisted Synthesis
Template-assisted synthesis involves the use of a sacrificial core material, such as silica nanoparticles, as a template for the growth of the metallic shell. The core particles are first functionalized with a coupling agent, such as aminopropyltriethoxysilane (APTES), to promote the adsorption of metal seeds. The metal shell is then grown by reducing a metal salt solution, such as chloroauric acid (HAuCl4), onto the seeded core particles. The thickness of the shell can be controlled by varying the concentration of the metal salt and the reaction time. After shell growth, the core material can be optionally etched away to obtain hollow nanoshells.
Galvanic Replacement Reaction
Galvanic replacement reaction is a simple and versatile method for preparing hollow nanoshells with tunable composition and porosity. In this method, a sacrificial metal nanoparticle template, such as silver nanoparticles, is oxidized and replaced by a more noble metal, such as gold, through a redox reaction. The driving force for the reaction is the difference in the reduction potentials of the two metals. By controlling the ratio of the sacrificial and noble metal precursors, the morphology and composition of the resulting nanoshells can be tuned. This method has been used to prepare gold-silver, platinum-silver, and palladium-silver nanoshells.
Laser Ablation Synthesis
Laser ablation synthesis is a physical method that uses a high-energy laser to ablate a bulk metal target in a liquid medium, producing metal nanoparticles. By performing the ablation in the presence of a dielectric material, such as silica or a polymer, core-shell nanoshells can be obtained. The laser parameters, such as wavelength, pulse duration, and fluence, can be adjusted to control the size and composition of the nanoshells. Laser ablation synthesis offers advantages such as high purity, minimal chemical waste, and the ability to produce nanoshells with complex compositions and structures.
Other nanoshell synthesis methods include chemical reduction, microemulsion synthesis, and self-assembly. The choice of the synthesis method depends on the specific requirements of the application, such as the desired optical properties, biocompatibility, and scalability. Ongoing research focuses on developing new synthesis strategies to improve the control over nanoshell size, shape, and composition, as well as to enable the large-scale production of high-quality nanoshells for biomedical applications.
The unique optical properties of nanoshells make them promising tools for various biomedical applications, including imaging, diagnostics, and therapy.
Optical Imaging and Diagnostics
Nanoshells with high scattering cross-sections can be used as contrast agents for optical imaging techniques, such as darkfield microscopy, reflectance confocal microscopy, and optical coherence tomography. By functionalizing the nanoshell surface with targeting ligands, such as antibodies or peptides, researchers can achieve molecular specificity and image specific biomarkers or disease sites. Nanoshells have been used to image cancer cells, monitor drug delivery, and detect biomolecular interactions.
Photothermal Therapy
Nanoshells with high absorption cross-sections can be used as photothermal agents for cancer therapy. When irradiated with NIR light, the nanoshells convert the absorbed energy into heat, causing localized temperature rise and thermal ablation of the surrounding tissue. By targeting nanoshells to tumor cells and selectively irradiating the tumor site, researchers can achieve highly specific and minimally invasive cancer treatment. Nanoshell-mediated photothermal therapy has shown promising results in preclinical studies and is being investigated in clinical trials.
Drug Delivery
Nanoshells can also serve as carriers for drug delivery applications. The metallic shell can be functionalized with drug molecules or polymers, allowing for controlled release of the therapeutic payload. Additionally, the photothermal effect of nanoshells can be used to trigger drug release or enhance the permeability of the target tissue, improving drug uptake and efficacy.
Comparison with Other Optical Imaging and Photothermal Agents
Nanoshells offer several advantages over other optical imaging and photothermal agents, such as organic dyes, gold nanorods, and carbon nanotubes.
Tunability
The LSPR wavelength of nanoshells can be easily tuned by varying the core-shell ratio, allowing for optimization of the optical properties for specific applications. This tunability is not readily achievable with other nanoparticles or dyes, which have fixed absorption and emission spectra.
Photostability
Nanoshells are highly photostable and resistant to photobleaching, unlike organic dyes or fluorescent probes. This photostability enables prolonged imaging and therapy sessions without significant signal loss or degradation.
Biocompatibility
Gold and silica, the main components of nanoshells, are generally considered biocompatible and have a long history of use in medical applications. The gold surface of nanoshells can be easily functionalized with biocompatible coatings or targeting ligands to improve their biological stability and specificity.
Challenges and Future Perspectives
Despite the promising potential of nanoshells in biomedical applications, several challenges need to be addressed for their clinical translation. These include the large-scale synthesis of uniform and reproducible nanoshells, the long-term stability and biodistribution of nanoshells in vivo, and the potential toxicity associated with the metallic shell. Ongoing research focuses on developing new synthesis methods, optimizing the surface chemistry and targeting strategies, and evaluating the safety and efficacy of nanoshells in preclinical and clinical studies.
Future directions in nanoshell research include the development of multifunctional nanoshells that combine imaging, therapy, and drug delivery capabilities, the integration of nanoshells with other nanotechnologies, such as magnetic nanoparticles or quantum dots, and the exploration of new applications beyond cancer, such as cardiovascular diseases, neurodegenerative disorders, and infectious diseases.
Further Reading
Advanced Functional Materials, Nanoshells with Targeted Simultaneous Enhancement of Magnetic and Optical Imaging and Photothermal Therapeutic Response
Cancer Nanotechnology, Nanoshells for Photothermal Cancer Therapy
Nature Reviews Materials, Hierarchical nanostructures: Nanoshells show their metal
