| Jul 02, 2019 | |
Improved fuel cell catalysts with less platinum(Nanowerk News) Scientists have identified highly active yet stable catalysts for use in fuel cells that contain only a quarter of the platinum as compared to existing devices (Science, "Ultralow-loading platinum-cobalt fuel cell catalysts derived from imidazolate frameworks"). |
|
| Platinum is essential for promoting reactions in these fuel cells. However, the precious metal is rare and expensive. Interactions between platinum-cobalt particles and a precious metal-free support contribute to the improved performance. | |
 |
|
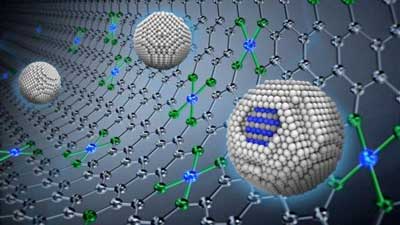
| An artistic rendition of the synergistic catalyst showing core-shell active sites (blue) in platinum-cobalt nanoparticles (spheres) on a platinum group metal-free catalytic support. (Image: Argonne National Laboratory) | |
| Proton-exchange membrane fuel cells (PEMFCs) are highly efficient. They represent a future powertrain for light and heavy-duty vehicles. A widespread transition from combustion engines to fuel cells in the automotive industry requires lower costs of materials and improved fuel cell performance. | |
| The catalysts identified in this study use a significantly lower amount of platinum, thus reducing cost. The new material also exceeds multiple 2025 DOE targets for fuel cells performance. | |
| Innovative approaches for reducing the amount of platinum in catalysts, while maintaining the high activity and stability that platinum provides, are of intense interest. The costly metal currently plays a crucial role in catalyzing multiple reactions in PEMFCs, including the breakdown of oxygen to form water. | |
| Scientists at Argonne National Laboratory’s Center for Nanoscale Materials (CNM), a DOE Office of Science user facility, are working with scientists and engineers at Argonne, Purdue University, and Shanghai Jiao Tong University to advance this research. | |
| After the team created the catalysts, the team characterized them using CNM’s aberration-corrected, high-resolution transmission electron microscope and spectroscopy capabilities at Argonne’s Advanced Photon Source, and modeled using CNM’s Carbon, a high-performance computing cluster. | |
| Together the team developed a synergistic catalytic system that involves platinum-cobalt nanoparticles and a platinum-group-metal-free (PGM-free) substrate. | |
| The synthesis involves converting a cobalt-containing porous metal-organic framework to a catalytically active substrate containing cobalt, nitrogen, and carbon decorated by nano-sized metallic crystallites, and then adding platinum to form platinum-cobalt nanoparticles with a core-shell structure that is confirmed by electron microscope measurements. | |
| These catalysts contain only about 3% of platinum by weight, in contrast to commercial fuel cell catalysts that contain 20% to 30% of platinum. The team evaluated the catalyst performance in operating PEMFC conditions. The results show significantly improved catalytic activity (with values of 1.08 to 1.77 A mgPt-1, exceeding the 2025 Department of Energy target of 0.44 A mgPt-1) and durability (retaining up to 64% of initial values after 30,000 voltage cycles). | |
| Computational modeling revealed the interaction between platinum-cobalt nanoparticles and PGM-free sites contribute to the improvements. |
| Source: U.S. Department of Energy, Office of Science | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
