| Aug 20, 2019 | |
Doped photovoltaics(Nanowerk News) Organic solar cells are made of cheap and abundant materials, but their efficiency and stability still lag behind those of silicon-based solar cells. |
|
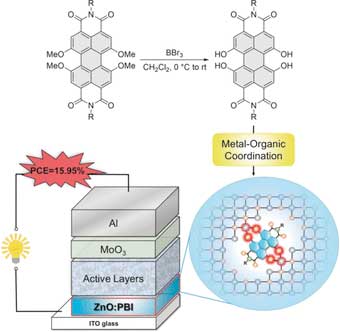
| A Chinese-German team of scientists has found a way to enhance the electric conductivity of organic solar cells, which increases their performances. Doping the metal oxide interlayer, which connected the electrode and active layer, with a modified organic dye boosted both the efficiency and stability, the study published in the journal Angewandte Chemie revealed ("Tetrahydroxy-Perylene Bisimide Embedded in Zinc Oxide Thin Film as Electron Transporting Layer for High Performance Non-Fullerene Organic Solar Cells"). | |
 |
|
| Organic solar cells convert light into electric current. The heart of the cells is the active organic layer made of specially designed organic molecules. Here, electrons and holes, the positive counterparts of the electrons, are generated by light and travel to the electrodes to form the electric current. | |
| A recurrent problem in organic solar cell design is the matching of the material types. The electrodes are made of inorganic materials, but the active layer is organic. To join the two materials, metal oxide interlayers are introduced in many organic cell types. But in most designs, the resulting conductivities are not optimal. | |
| Frank Würthner at the University of Würzburg, Germany, and Zengqi Xie at the South China University of Technology (SCUT), Guangzhou, China, investigated the idea of making a zinc oxide interlayer slightly more organic and photoconductive to reduce the contact resistance when irradiated with sunlight. | |
| The scientists prepared an organic dye in such a way that it formed stable complexes with the zinc ions present in the zinc oxide layer. Under sunlight, this modified dye called hydroxy-PBI would then inject electrons into the zinc oxide interlayer, which would increase its conductivity. | |
| The scientists then assembled the organic solar cell, which consisted of an indium tin oxide glass (ITO) electrode, the zinc oxide layer doped with the hydroxy-PBI dye, the active layer made of a polymer as the electron donor and an organic molecule as the acceptor, another metal oxide interlayer, and an aluminum electrode as the positive electrode. | |
| This architecture, which is called an inverted bulk heterojunction cell, is that of a state-of-the-art organic solar cell, which achieves a maximum 15 percent power conversion efficiency. | |
| The interlayer doping was beneficial in several ways. Depending on the dye—the scientists checked the performance of several dyes with slightly different structures— conversion efficiencies of almost 16 percent were achieved. And the dye-doped zinc oxide interlayer also appeared to be more stable than one without the doping. | |
| The authors said that it was important that the PBI dye was modified to its hydroxy-PBI form, which gave rise to tight complexes with the zinc ions. Only then could an inorganic–organic hybrid structure evolve to form a good contact with the active materials. |
| Source: Wiley | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
