| Nov 26, 2019 | |
Smart contrast agent for tumour visualisation(Nanowerk News) National University of Singapore (NUS) chemists have developed a high performance contrast agent for magnetic resonance imaging (MRI) technology to improve cancer diagnosis (Advanced Materials, "In vivo tumor visualization through MRI off-on switching of NaGdF4-CaCo3 nanoconjugates"). |
|
 |
|
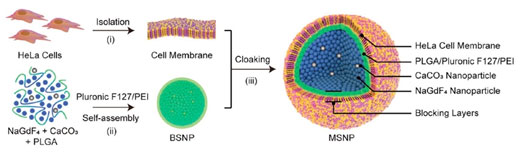
| Figure illustrates the formation of the cell-membrane self-assembled nanoparticles (MSNP). The NaGdF4 and CaCo3 nanoparticles together with polymer linkers self-assemble themselves into spherical shapes due to chemical interactions between them. These nanoparticles are then coated with cell membrane extracted from laboratory cultivated HeLa cells. [© Advanced Materials] (click on image to enlarge) | |
| MRI technology is a non-invasive medical imaging technique for disease diagnosis and therapeutic monitoring. It uses chemical compounds known as contrast agents to improve the contrast between the internal body structures and surrounding tissues. | |
| Commercially available contrast agents for MRI suffer from two main drawbacks. They are not able to bind specifically onto cancerous tumours and have a short circulation time within the body. The signal-to-noise ratio is often low and this results in MRI images which have poor spatial resolution. | |
| It remains challenging to develop biocompatible contrast agents which can target cancerous tumours (with high selectivity) and provide imaging signals with high signal-to-noise ratios so that higher quality images can be obtained for medical diagnostic purposes. | |
| A research team led by Prof LIU Xiaogang from the Department of Chemistry, NUS has developed a new class of nanoparticle-based contrast agents, known as MSNPs using self-assembled NaGdF4 and CaCo3 nanoparticles. | |
| Using a self-assembly process, the NaGdF4 and CaCo3 nanoparticles are agglomerated into spherical cores. Each of these cores is encapsulated by a layer of lab cultivated cell membrane. The MSNPs are found to exhibit good biocompatibility and high tumour selectivity. Under normal conditions in the body, the MSNP is not activated and gives an “off” signal. | |
| However, when the microenvironment is mildly acidic, which is the case for most cancer tumours, it becomes activated. Under this condition, the CaCo3 compound in the MSNPs reacts with water molecules present in the body to form carbon dioxide bubbles. This causes the disintegration of the MSNPs, releasing the NaGdF4 nanoparticles into the surrounding cancerous tissues. | |
| This results in an “on” signal. Based on experimental results using murine models, the MSNPs show a contrast enhancement of potentially more than 60 fold improvement in tumour visualisation when compared to Magnevist, a commercially available MRI contrast agent. | |
| Prof Liu said, “These research findings may improve the capability of medical imaging technologies by providing a pH-responsive contrast agent that is potentially more sensitive and selective than commercially available products.” | |
| The research team plans to further develop and refine the design of their nanoparticles by incorporating variations to cater to a wider range of chemical dynamics and expand their potential applications in molecular sensing and medical imaging technologies. |
| Source: National University of Singapore | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
