| Dec 04, 2019 | |
Novel material switches between electrically conducting and insulating states(Nanowerk News) Northwestern Engineering researchers have developed a novel design strategy to identify new materials exhibiting a metal-insulator transition (MIT), a rare class of materials categorized by their ability to reversibly switch between electrically conducting and insulating states. |
|
| The new method could jumpstart future design and delivery of faster microelectronics with more storage capabilities, as well as quantum materials platforms for future electronics. | |
| "Our approach uses anion substitution at the atomic scale and the recognition of key MIT properties to identify potential heteroanionic MIT materials, which have not been widely considered to this point," said James Rondinelli, associate professor of materials science and engineering and the Morris E. Fine Junior Professor in Materials and Manufacturing at the McCormick School of Engineering, who led the team. "We hope by formulating these electronic structure-property relationships, new transitions in quantum materials can be designed in the future." | |
| A paper outlining the work was published in the journal Physical Review Letters ("Design of Heteroanionic MoON Exhibiting a Peierls Metal-insulator Transition"). Rondinelli was the paper's co-corresponding author along with Danilo Puggioni, a research assistant professor in the Department of Materials Science and Engineering. | |
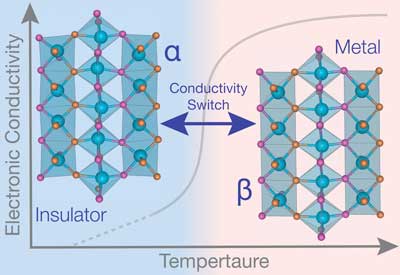 |
|
| Researchers found the metal-insulator transition in the material molybdenum oxynitride occurred near 600 degrees Celsius, revealing its potential for applications in high-temperature sensors and power electronics. (Image: Northwestern University) | |
| Using quantum-mechanical computer simulations at Northwestern's Quest High Performance Computing Cluster, Rondinelli and researchers designed the picoscale crystalline structure of the new material, called molybdenum oxynitride (MoON), to host the phase transition. The researchers found the MIT occurred near 600 degrees Celsius, revealing its potential for applications in high-temperature sensors and power electronics. | |
| The group noted multiple design parameters influenced MoON's phase transition. The inclusion of multiple anions in the material -- in this case, negatively charged oxygen and nitrogen ions -- activated the phase transition due to specific electron configurations related to the spatial orientation of electronic orbitals, supporting previous findings in other binary MIT materials. In addition, MoON's flexible rutile crystal structure lent reversibility between electrically conducting and insulating states. | |
| The findings offer insight into how subtle changes on the nanoscale can be used to control macroscopic behavior -- like conductivity -- in materials. | |
| "Substantial work has been done during the past decade to understand MIT materials and discover new ones; however, less than 70 unique compounds are currently known that exhibit this thermal transition," Rondinelli said. "We embodied key features of MIT materials, including particular picoscale structural features, as well as the crucial d1 electron configuration, into our design. Our project leverages a way that we and others can use key first-principle design concepts to expand the MIT phase space and effectively pursue new MIT materials." | |
| Scientists hope by formulating these electronic structure-property relationships, new transitions in quantum materials can be designed in the future. These compounds are useful as the active layer for transistors or in memory applications. | |
| "MIT materials represent a class of phase transitions that may enable advances in information processing and storage beyond conventional complementary metal-oxide semiconductor scaling in microelectronics," Rondinelli said. "This translates to faster devices with more storage capabilities. In addition, MIT materials could enable low?power microelectronic systems, meaning you would need to charge your device less frequently, as it lasts longer because the components require less power." |
| Source: Northwestern University | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
