| May 20, 2020 | |
Attosecond physics: Quantum brakes in molecules(Nanowerk News) Physicists of LMU Munich have measured the flight times of electrons emitted from a specific atom in a molecule upon excitation with laser light. This has enabled them to measure the influence of the molecule itself on the kinetics of emission. |
|
| Photoemission – the release of electrons in response to excitation by light – is one of the most fundamental processes in the microcosm. The kinetic energy of the emitted electron is characteristic for the atom concerned, and depends on the wavelength of the light employed. But how long does the process take? And does it always take the same amount of time, irrespective of whether the electron is emitted from an individual atom or from an atom that is part of a molecule? | |
| An international team of researchers led by laser physicists in the Laboratory for Attosecond Physics (LAP) at LMU Munich and the Max Planck Institute of Quantum Optics (MPQ) in Garching has now probed the influence of the molecule on photoemission time (Nature Physics, "Probing molecular environment through photoemission delays"). | |
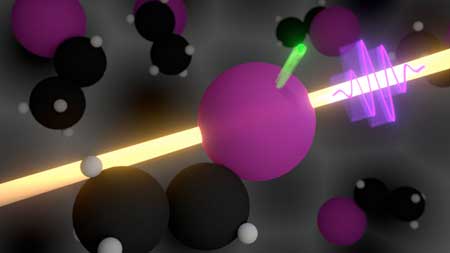 |
|
| An ultrashort x-ray laser pulse (in violet) removes an inner-shell electron from the iodine atom in ethyl iodide. The experiment times the propagation of the electron with attosecond precision, and measures how much the released electron is decelerated or accelerated by intramolecular forces. (Graphics: Philipp Rosenberger) | |
| The theoretical description of photoemission in 1905 by Albert Einstein marked a breakthrough in quantum physics, and the details of the process are of continuing interest in the world of science and beyond. How the motions of an elementary quantum particle such as the electron are affected within a molecular environment has a significant bearing on our understanding of the process of photoemission and the forces that hold molecules together. | |
| In close collaboration with researchers from the King Saud University (KSU) in Riyadh (Saudi Arabia), and additional international partners, the team at LAP has now determined how long it takes electrons to be photo-emitted from a specific atom within a molecule (in this case, the iodine in ethyl iodide). The measured times were in the range of tens of attoseconds. One attosecond is a billionth of a billionth of a second. | |
| The researchers used a range of pulses in the x-ray region to excite the targeted electron. The use of machine learning helped to improve the precision of the analysis of the experimental data, and resulted in more accurate comparisons with theoretical predictions. | |
| “The comparison of the experimental data with theoretical simulations finally revealed the influence of the molecule on the time that electrons need for the photoemission process,” explains Professor Matthias Kling, who heads the Ultrafast Imaging and Nanophotonics group within the LAP team. | |
| The researchers found that the delay attributable to the molecular environment became larger as the energy of the light pulses – and hence the initial kinetic energy imparted to the electrons – was reduced. | |
| The observations may be compared with exploring a landscape. When flying over it, many details on the ground remain unnoticed. At ground level, every single bump makes itself felt. The same is true for excited electrons. If the initial impulse is just enough to enable them to leave the molecule, the retarding effect of the forces that hold the molecule together is greater than when the ‘kick’ is sufficiently energetic to eject them more promptly. | |
| “Our observations indicate that experiments tracing photoemission time permit us to learn about the forces within molecules”, explains Professor Abdallah Azzeer, Head of the Laboratory for Attosecond Physics at KSU in Riyadh. “These studies could improve our understanding of quantum effects in molecules and chemical reactions,” adds Prof. Alexandra Landsman from Ohio State University in the US, who leads the group that conducted the majority of the theoretical work. |
| Source: By Thorsten Naeser, LMU | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
