| Mar 12, 2021 |
Peering into bacteria with X-ray nanovision
(Nanowerk News) Whether looking inside human bodies, luggage, or other applications, most X-ray scans are two dimensional. They work by measuring the transmission of X-rays through a material, like dental X-rays of the teeth. Three dimensional images are much more informative. These images are taken in three dimensions and yield a computed tomography scan, commonly known as a “CT scan.”
|
|
Standard X-ray machines provide a picture with a few millimeters resolution at best. However, X-ray beams from a synchrotron provide the possibility of “seeing” much smaller objects. A synchrotron accelerates electrons to almost the speed of light to create extremely bright light.
|
|
These special X-ray beams can peer into molecules in biological cells or other materials. Bio-molecules are nearly invisible to X-rays, so researchers must attach a molecular tag to the molecules that glows when illuminated with X-rays.
|
|
In this study (JACS, "Lanthanide-Binding Tags for 3D X-ray Imaging of Proteins in Cells at Nanoscale Resolution"), scientists used an X-ray-sensitive tag illuminated by a tiny synchrotron X-ray beam, whose cross-section was 1,000 times smaller than a thinnest human hair. Using this technique, they created an image of a membrane protein on the surface of a single E. coli bacteria.
|
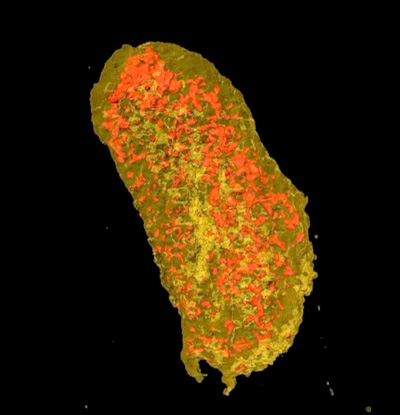 |
| A nanoscale X-ray scan of an E.coli bacteria. Yellow represents the membrane protein, which lights up with X-rays due to a specialized tag attached to the protein. Red represents zinc, which is important for reproduction. (© JACS)
|
|
Biological cells are surrounded by barriers called lipid bilayers. Membrane proteins in these barriers act as gateways, allowing ions, drugs, and other molecules to pass in and out of cells. These gateways are often the target of drug treatments to kill cells in cases of infections or cancer. This new X-ray nanovision technology allows scientists to “see” cell membrane proteins and watch how they operate.
|
|
These new X-ray nano-CT scans will aid in discovery of new membrane protein targets for wide ranging applications, including the next generation of drugs for combatting diseases. Scientists can also use the scans to study the molecular structure of membranes in batteries, fuel cells, and other systems. The technique will help researchers understand molecules in action in these important energy technologies.
|
|
Membrane proteins are responsible for the transport of ions—including calcium, sodium, and potassium—in and out of cells. Ion transport is also essential to batteries and similar energy technologies. In biological systems, the regulation of ion transport is important for all life and for preventing a wide range of diseases including epilepsy and cystic fibrosis.
|
|
Thus, membrane proteins are targets for drugs to combat such diseases. For example, the “spike protein” on the SARS-CoV-2 virus is a membrane protein that is a primary target for fighting COVID-19. To see membrane proteins in cells, scientists employ a specialized microscope because the protein molecules are so small—10,000 times smaller than a human hair.
|
|
Only recently have synchrotron X-ray beams been made small enough for this task. However, one major problem is that proteins are nearly invisible to X-rays.
|
|
To solve this problem, scientists have taken an X-ray-sensitive “tag”—a tracer molecule that lights up when illuminated by X-rays—and fused it to a membrane protein so it can be visualized with the tiny X-ray beam. Using a nano-sized X-ray beam at the National Synchrotron Light Source II (NSLS-II) and the Advanced Photon Source, both Department of Energy user facilities, the researchers obtained the highest resolution CT scans ever achieved of a membrane protein in a cell.
|
|
Since the fusion of these tags to membrane proteins follows a straightforward biochemistry lab protocol, it is possible that synchrotron nano-CT could become a widespread tool for 3-D imaging of many different proteins in cells at the nanoscale.
|
|
Moreover, since X-rays have deep penetrating power, this method can be extended beyond imaging single cells in a lab petri dish to studying cells within their natural tissue environment.
|

