| Mar 25, 2021 |
Researchers discover new organic conductor
(Nanowerk News) Salts are far more complicated than the food seasoning - they can even act as electrical conductors, shuttling current through systems. Extremely well studied and understood, the electrical properties of salts were first theorized in 1834. Now, nearly 200 years later, researchers based in Japan have uncovered a new kind of salt.
|
|
The results were published in Inorganic Chemistry ("Tetramethyltetrathiafulvalene [(NbOF4)-]∞: One-Dimensional Charge Transfer Salt with an Infinite Anion Chain").
|
|
The researchers were specifically investigating how one-dimensional versions of three-dimensional substances exhibit unique physical phenomena and functionality in a process called the phase diagram.
|
|
"We were developing a new substance to deepen our understanding of the phase diagram," said paper author Toshikazu Nakamura, researcher with the Institute for Molecular Science of the National Institutes of Natural Sciences. "In this process, I found a completely new salt."
|
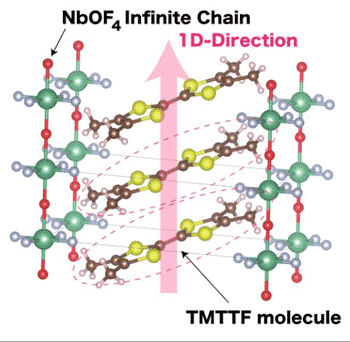 |
| Crystal Structure of One-Dimensional Charge Transfer Salt with an Infinite Anion Chain. (Image: NINS/IMS)
|
|
Tetrathiafulvalene is a sulfuric compound that acts as a skeleton for several organic conductor salts. Its molecular structure can be built upon to develop new substances, and it can be easily tweaked to adjust the structural parameters as part of the phase diagram. Nakamura was building upon this compound with negatively charged ions and an atomic group derived from carbon disulfide. During this process, the one-dimensional substance transferred an electric charge, and converted into an entirely new material.
|
|
Conventional organic conductors have an easily deformed lattice structure and are composed of more complicated arrangements, according to Nakamura. The new conductor's negative ions are arranged with an infinite chain structure, stabilizing the atomic arrangements of niobium, oxygen and fluorine. When exposed to low temperatures of 5 Kelvin, about -450 degrees Fahrenheit, neighboring sites on the salt begin to develop a magnetic coordination.
|
|
"We will investigate this phenomenon in detail -- we want to understand the origin," Nakamura said.
|
|
The researchers plan to study other infinite chain salts with the goal of understanding and applying the structure as the skeleton of new organic conductors as one-dimensional electronic systems.
|
|
"Our ultimate goal is to understand the electronic state of these systems and what happens when we gradually increase the inter-chain interactions from one dimension to two dimensions," Nakamura said.
|

