| Jan 25, 2022 |
Asymmetry is key to creating more stable blue perovskite LEDs
(Nanowerk News) From street and household lighting, to television and mobile displays, light emitting diodes (LEDs) play an essential role in modern life. Now, researchers from the Okinawa Institute of Science and Technology Graduate University (OIST) have developed blue LEDs based on a material called metal halide perovskite, that, for the first time, uses asymmetrical bridges to hold the layers of perovskite together, creating a more stable structure.
|
|
The study, published recently in the Journal of the American Chemical Society ("Spectral Stable Blue-Light-Emitting Diodes via Asymmetric Organic Diamine Based Dion–Jacobson Perovskites"), could bring perovskite LEDs one step closer to commercialization.
|
 |
| Researchers from the OIST Energy Materials and Surface Sciences Unit have created blue LEDs that use a new structure to enhance stability. (Image: OIST)
|
|
“Perovskites have the potential to be a real game-changer in the lighting industry,” said first author Dr. Yuqiang Liu, a former post-doctoral researcher in the OIST Energy Materials and Surface Sciences Unit and currently a professor at Qingdao University, China. “In only a few short years, the efficiency of perovskite LEDs – how well they can transfer electrical energy into light energy – has shot up to a level that rivals traditional LEDs, and soon will surpass them.”
|
|
Aside from efficiency levels, perovskite LEDs also have numerous advantages over current LED technologies on the market, as they have the potential to produce brighter, purer colors at a fraction of the production cost.
|
|
However, the stability of perovskite LEDs remains a huge barrier, with the operational lifetime of even the most stable LEDs lasting only a few hundred hours. Blue LEDs, in particular, have lagged behind red and green-colored LEDs, with a lifetime of less than 2 hours and around half the level of efficiency.
|
|
But without blue LEDs, practical applications using perovskites in color displays or as light sources are limited, as red, green and blue light need to be mixed to produce the full array of colors, including white, explained Professor Yabing Qi, senior author of the paper and head of the OIST Energy Materials and Surface Sciences Unit.
|
|
“Historically, blue emission has always been much more difficult to achieve,” Prof. Qi continued. “The Nobel prize-winning blue LEDs that were first made using gallium nitride took three decades longer to develop than red and green LEDs – and even now, creating large, high-quality crystals of gallium nitride remains challenging and expensive. So, there is very much a need for research into new blue-emitting materials, like perovskites.”
|
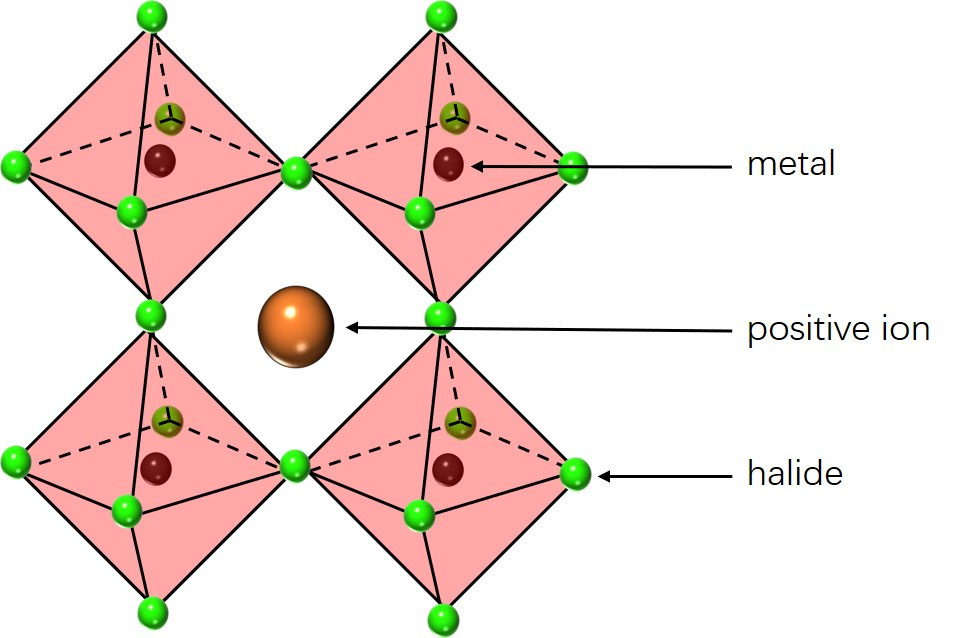 |
| In the crystal structure of a metal halide perovskite, halide ions (small green balls) create an octahedral shape (pink) around a metal atom (dark red ball). In the middle of a group of four octahedral shapes lies a positive ion (orange ball). (Image: OIST)
|
|
In the study, the scientists looked at one of the major issues seen in blue perovskite LEDs – the halide segregation problem.
|
|
When metal halide perovskite crystals form, the halides bond in an octahedral shape around a metal atom. A positive ion is situated in between four of these octahedral shapes.
|
|
However, when a voltage is applied across a perovskite LED, which is required for the LED to emit light, it also causes the negative halide ions that form the octahedral structure to separate and migrate towards the positive end of the LED. The positive ions between the octahedral shapes also migrate to the negative end of the LED. This ion migration degrades the perovskite structure, causing the efficiency of the LED to plummet and the blue color to shift to a greener hue.
|
|
To try and combat the halide segregation problem, the researchers created blue LEDs with a type of perovskite structure called a Dion-Jacobson phase structure, where two-dimensional (2-D) layers of perovskite crystal are stacked on top of each other. The perovskite layers are then linked together by molecular bridges, increasing the stability of the whole structure.
|
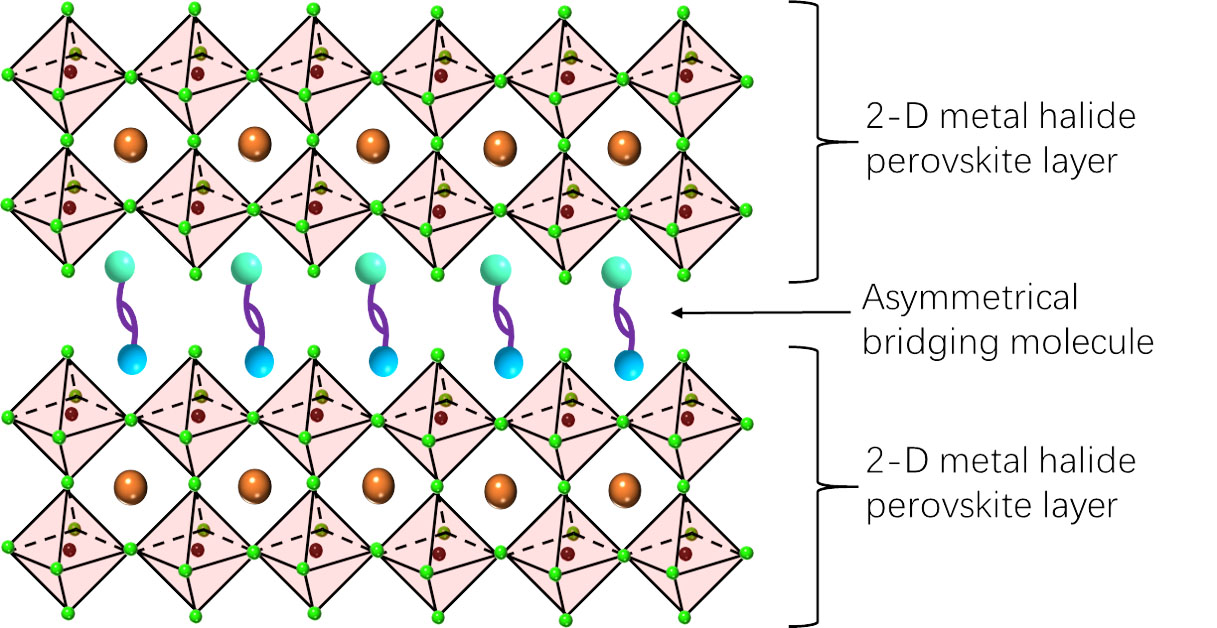 |
| The researchers created a perovskite structure where 2-D layers of perovskite were linked by asymmetrical bridging molecules. (Image: OIST)
|
|
In previous research, the molecular bridges that were created were symmetrical, which means that both ends of the molecule looked the same.
|
|
Now, for the first time, the researchers explored whether using an asymmetrical bridge, where each end was different, affected the overall properties of the perovskite LED.
|
|
The researchers found that when the bridging molecule was asymmetrical, it slowed down the migration of ions across the layers of perovskite, therefore improving the stability of the perovskite structure.
|
|
The team proposed that the asymmetry causes changes in how the electrons are distributed across the bridging molecule, therefore creating a small dipole electric field in between the layers.
|
|
“We think this dipole electric field is what is interfering with the ion migration, and therefore maintaining stability,” said Prof. Qi.
|
|
As well as solving the problem of halide segregation in perovskite LEDs, the strategy of using asymmetrical bridges could also be applied to other perovskite-based devices, such as perovskite solar cells.
|
|
“It’s an exciting advancement towards creating all kinds of longer-lived perovskite devices,” Prof. Qi concluded.
|



