| Jan 31, 2023 |
Converting temperature fluctuations into clean energy with novel nanoparticles and heating strategy
(Nanowerk News) Pyroelectric catalysis (pyro-catalysis) can convert environmental temperature fluctuations into clean chemical energy, like hydrogen. However, compared with the more common catalysis strategy, such as photocatalysis, pyro-catalysis is inefficient due to slow temperature changes in the ambient environment.
|
|
Recently, a team co-led by researchers at City University of Hong Kong (CityU) triggered a significantly faster and more efficient pyro-catalytic reaction using localized plasmonic heat sources to rapidly and efficiently heat up the pyro-catalytic material and allow it to cool down. The findings (Nature Communications, "Accelerated pyro-catalytic hydrogen production enabled by plasmonic local heating of Au on pyroelectric BaTiO3 nanoparticles") open up new avenues for efficient catalysis for biological applications, pollutant treatment and clean energy production.
|
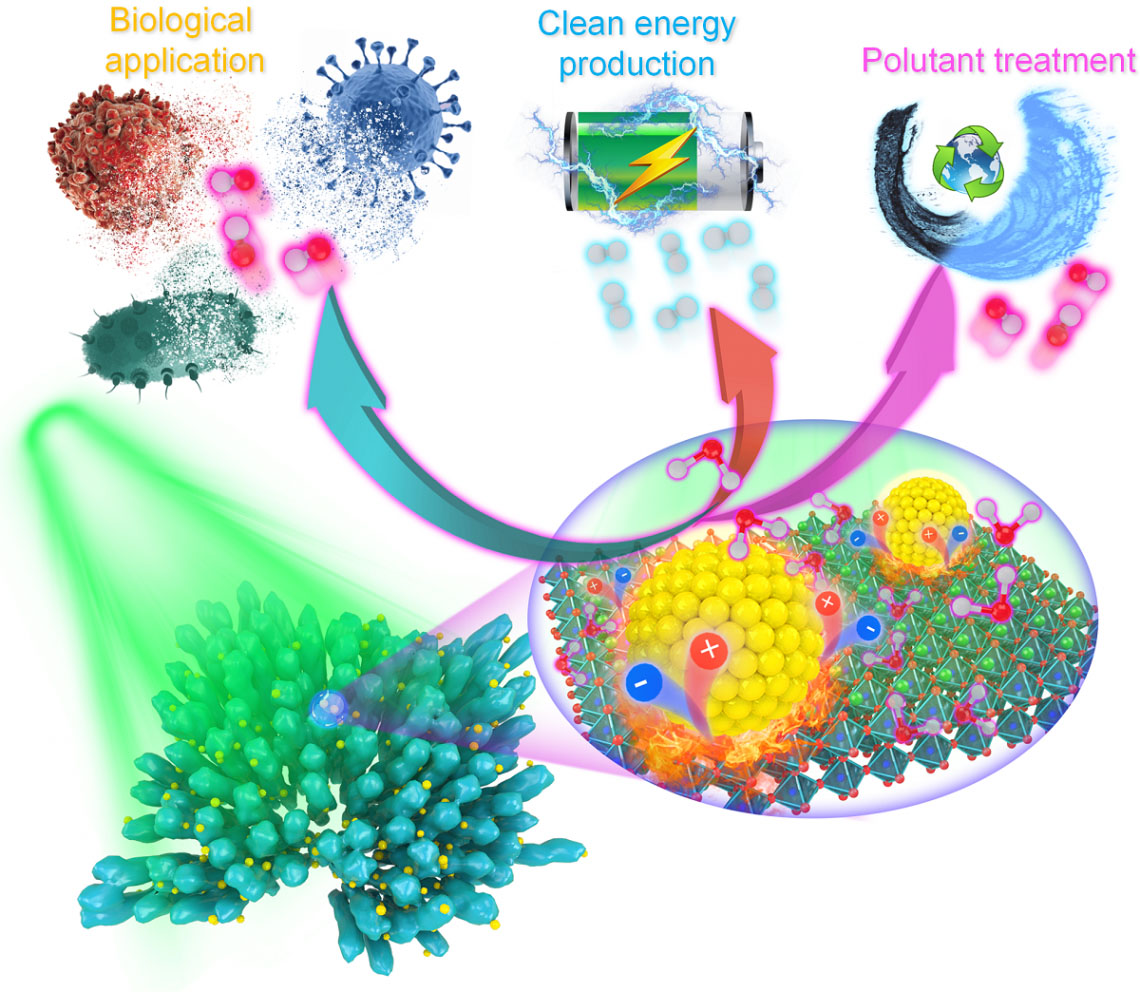 |
| Illustration of potential applications of combining pyroelectric materials and the localized thermo-plasmonic effect of noble metal nanomaterials. (Image: Dr Lei Dangyuan’s group / City University of Hong Kong)
|
|
Pyro-catalysis refers to the catalysis triggered by surface charges in pyroelectric materials induced by temperature fluctuations. It is a green, self-powered catalysis technique, which harvests waste thermal energy from the environment. It has attracted increasing attention in clean energy production and reactive oxygen species generation, which can be further used for disinfection and dye treatment.
|
|
However, most of the currently available pyroelectric materials are not efficient if the ambient temperature doesn’t change much over the time. As the environmental temperature change rate is often limited, a more viable way to increase the pyro-catalytic efficiency is to increase the number of temperature cycling. But it is a great challenge to achieve multiple thermal cycling in the pyro-catalyst within a short time interval using conventional heating methods.
|
Challenge of multiple thermal cycling
|
|
A research team co-led by Dr Lei Dangyuan, Associate Professor in the Department of Materials Science and Engineering (MSE) at CityU, recently overcame this obstacle using a novel strategy of combining pyroelectric materials and the localized thermo-plasmonic effect of noble metal nanomaterials.
|
|
The plasmonic nanostructures, which support the collective oscillation of free electrons, can absorb light and convert it quickly into heat. Its nanoscale size allows fast yet effective temperature changes within a confined volume, without significant heat loss to the surrounding environment. Consequently, the localized heat generated by the thermo-plasmonic nanostructures can be easily fine-tuned and turned on or off by external light irradiation within an ultrashort time interval.
|
|
In their experiments, the team selected a typical pyro-catalytic material, called barium titanate (BaTiO3) nanoparticles. The coral-like BaTiO3 nanoparticles are decorated with gold nanoparticles as plasmonic heat sources; the gold nanoparticles can convert the photons directly from a pulsed laser to heat. The experiment results demonstrated that gold nanoparticles act as a rapid, dynamic and controllable localized heat source without raising the surrounding temperature, which prominently and efficiently increase the overall pyro-catalytic reaction rate of BaTiO3 nanoparticles.
|
Gold nanoparticles as a localized heat source
|
|
Through this strategy, the team achieved a high pyro-catalytic hydrogen production rate, speeding up the practical application development of pyro-catalysis. The plasmonic pyroelectric nano-reactors demonstrated an accelerated pyro-catalytic hydrogen production rate of about 133.1±4.4 µmol·g-1·h-1 through thermo-plasmonic local heating and cooling under irradiation of a nanosecond laser at the wavelength of 532 nm.
|
|
Furthermore, the repetition rate of the nanosecond laser used in the experiment was 10 Hz, which meant that 10 pulses of light were irradiated on the catalyst per second to achieve 10 heating and cooling cycles. This implies that by increasing the laser pulse repetition rate, the pyroelectric catalytic performance could be improved in the future.
|
|
The research team believes that their experiment results have opened up a new approach for improving pyro-catalysis by designing an innovative pyroelectric composite system with other photothermal materials. This substantial progress will make the future application of pyro-catalysis in pollutant treatment and clean energy production more feasible.
|

