| Jun 06, 2023 |
Gold nanoparticles harness energy from the sun to break down pollution
(Nanowerk News) When organic pollutants such as dyes, agricultural chemicals, and pharmaceuticals enter waterways all around the world, they can harm the environment and human health – and removing them can be incredibly difficult.
|
|
Photocatalysts – substances that absorb energy from light and use it to accelerate the rate of a chemical reaction – can decompose organic pollutants in a process called mineralization, converting them into water, carbon dioxide, and other harmless molecules. But there’s a catch: most photocatalysts require UV light to work, making them impractical and expensive to use at scale.
|
|
To solve that problem, researchers have their sights set on finding a photocatalyst that can harness much more of the solar spectrum. “If you can use solar light, it’s cheaper and much more available than UV light,” says Magnus Rønning, a professor in catalysis at the Department of Chemical Engineering, NTNU.
|
Gold nanoparticles key
|
|
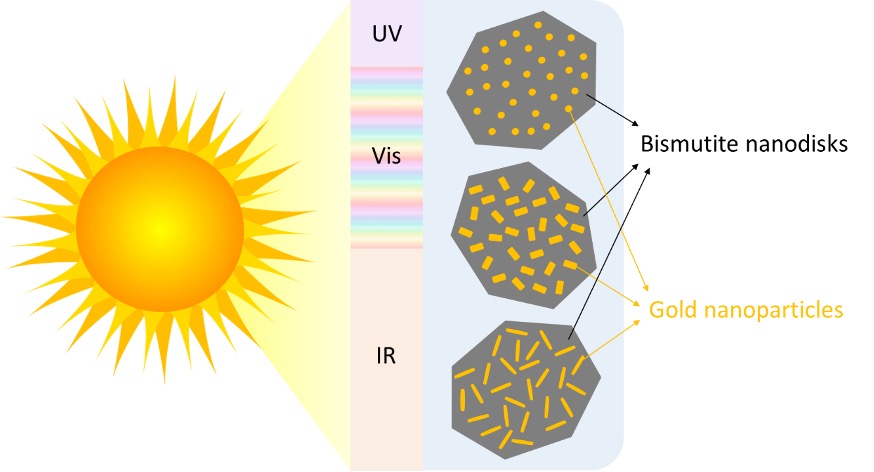
Nano-sized disks made of the mineral bismutite are a promising photocatalyst, and researchers have discovered that adding gold nanoparticles to their surface increases their sensitivity to the visible part of the solar spectrum. However, while there are several ways to deposit those gold nanoparticles on a surface, most methods give limited control over the size and shape of the particles you end up with.
|
|
“Often you will get a distribution of sizes and shapes [that] you cannot really control, so you have a mix of rods and spheres and cubes,” says Rønning.
|
|
Now, Rønning and colleagues at NTNU have found a way to create gold nanoparticles with a uniform size and shape on the surface of the bismutite nanodisks. Their research, recently published in the journal Photochemical and Photobiological Sciences ("Optimizing the shape anisotropy of gold nanoparticles for enhanced light harvesting and photocatalytic applications"), opens up the possibility of studying the effect of both the size and shape of the nanoparticles on the performance of the catalyst, in turn making it possible to maximize its light-harvesting capacity.
|
 |
| Optimizing the shape anisotropy of gold nanoparticles for enhanced light harvesting and photocatalytic applications.
|
Tested different shaped nanoparticles
|
|
The researchers used small gold seeds as nucleation sites on which they grew gold nanoparticles in different shapes – rods, etched rods with roughened surfaces, and spheres – by adjusting the concentration and pH of the solution the particles were growing in.
|
|
These nanoparticles have a surfactant layer on their surface, which reduces the likelihood they will clump together, before they are deposited on the bismutite nanodisks.
|
|
“With this, we can keep a reasonably good control over the size and shape of these particles,” says Rønning. The samples were prepared and characterized by PhD candidate Jibin Antony in NTNU’s NanoLab, with the catalytic reactions themselves run in the labs of the Catalysis Group in the Department of Chemical Engineering.
|
Promising tests
|
|
The researchers tested how well the resulting photocatalyst could break down an organic pollutant known as methylene blue. As well as being a widespread organic contaminant in its own right, methylene blue is a useful test case for how well a photocatalyst will work on other pollutants.
|
|
“It’s quite representative as an organic contaminant, but it’s also a relatively complex molecule,” says Rønning. “If it works on methylene blue, it should also work well on other organics.”
|
|
The other advantage of using methylene blue is that its decomposition is already well understood, allowing the researchers to probe not just how much methylene blue is left at the end of the process, but what it’s been converted to.
|
|
While that is not something that Rønning and colleagues looked at in their work on gold nanoparticles, in a related paper the researchers saw that adding silica to bismutite nanodisks did change the degradation products of methylene blue. “In the end, you want full mineralization and not just conversion into something that is just as dangerous or unwanted as the methylene blue,” says Rønning.
|
Rods better than spheres
|
|
Rønning and his colleagues found that the photocatalyst with rod-shaped gold nanoparticles performed 14% better than the one with spheres. But there is still room for improvement. “Even after three hours of reaction, you still have some of the contaminant left,” says Rønning. “So, yes, it’s working. But we still need something that works better.”
|
|
Some photocatalysts are already used in wastewater treatment and air purification systems commercially. The technology also holds promise for hydrogen splitting – producing cheap hydrogen fuel using just water and sunlight. However, to make that possible, researchers need to find a way to make the catalysts much more effective than they are today.
|
|
The key improvement needed for better photocatalysts rests on how many of the photons are actually used to drive the reaction. “In good cases, it’s maybe 1%,” says Rønning. “If we can get this up to, say, 10%, it will be much closer to practical applications.”
|
|
While changing the size and shape of gold nanoparticles is unlikely to bring about such a huge increase in efficiency, it’s a start. “We need to improve the catalyst in order for this to be commercially viable,” says Rønning. “This is a step in that direction.”
|

