| Sep 12, 2017 |
Artificial enzymes for hydrogen conversion
|
|
(Nanowerk News) Cell Press has just released the first issue of Joule, a new journal dedicated to sustainable energy research. In this issue James Birrell, Olaf Rüdiger, Edward Reijerse and Wolfgang Lubitz, from the Max Planck Institute for Chemical Energy Conversion, summarize the development of artificial maturation of hydrogenases and how this invention has opened up new avenues in the study of these enzymes, and describe the impact of these findings on energy research in the future (1).
|
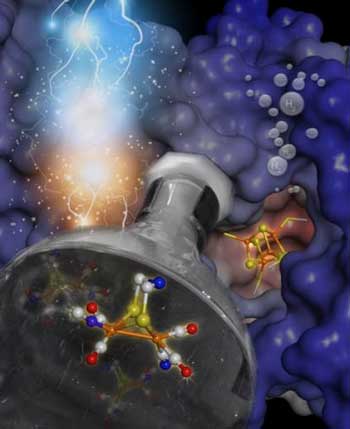 |
| Chemistry meets biology: synthetic inorganic complexes can be incorporated into hydrogenases to make active hydrogen producing semisynthetic enzymes. (Image: MPI CEC)
|
|
James Prescott Joule, eponym of this new journal, could have been describing the hydrogenases when he stated “The animal frame … is as a machine more perfect than the best contrived steam-engine—that is, is capable of more work with the same expenditure of fuel” (2), clearly a reference to the exemplary efficiency of biological energy conversion processes over those contrived by hu-man invention.
|
|
As researchers, we constantly try to learn from nature. The development of inexpensive, stable, efficient and highly active catalysts for reversible hydrogen conversion will allow us to generate and use hydrogen as an energy currency in a future society free from fossil fuels.
|
|
Hydrogenases are natural catalysts displaying superb activity and efficiency in the hydrogen conversion reaction. The generation of semisynthetic hydrogenases using artificial maturation of the active site represents a milestone in bioenergy research (3,4). It allows the enzymes to be produced recombinantly in high yields and purity, and combined with chemically synthesized active site cofactors.
|
|
These cofactors can be chemically different from the native one allowing specific alterations of its catalytic properties, or isotopically labeled for spectroscopic studies, which provide crucial insight into the mechanism of the hydrogenases. This is important for designing new molecular catalysts for hydrogen conversion using cheap and abundant metals like iron.
|
|
This approach, developed for hydrogenases, can be extended to design scaffolds for housing other molecular catalysts and tuning their properties, e.g. for other important energy conversion reactions such as N2 or CO2 fixation.
|
References
|
|
1. Birrell, J.A., Rüdiger, O., Reijerse, E.J., Lubitz, W. (2017) Semisynthetic hydrogenases propel biological energy re-search into a new era. Joule 1, 61–76
|
|
2. James Prescott Joule (1884). On matter, living force, and heat. In The scientific papers of James Prescott Joule.
|
|
3. Berggren, G., Adamska, A., Lambertz, C., Simmons, T.R., Esselborn, J., Atta, M., Gambarelli, S., Mouesca, J.M., Reijerse, E., Lubitz, W., Happe, T., Artero, V., and Fontecave, M. (2013). Biomimetic assembly and activation of [FeFe]-hydrogenases. Nature 499, 66-69.
|
|
4. Esselborn, J., Lambertz, C., Adamska-Venkatesh, A., Simmons, T., Berggren, G., Noth, J., Siebel, J., Hemschemeier, A., Artero, V., Reijerse, E., Fontecave, M., Lubitz, W., and Happe, T. (2013). Spontaneous activation of [FeFe]-hydrogenases by an inorganic [2Fe] active site mimic. Nat. Chem. Biol. 9, 607-609.
|

