| Jun 19, 2018 |
Artificial biosynthesis yields superior steroid antibiotics
|
|
(Nanowerk News) Researchers at the University of Tokyo discovered that they had obtained supernatural analogs of steroid antibiotics from a system they constructed for the microbial production of the antibiotics. The analogs have better antibiotic activity than compounds synthesized through conventional natural methods.
|
|
The results may pave the way for the application of synthetic biology methods toward drug development by offering a scaffold for pharmaceutically important molecules.
|
|
Natural products have attracted much interest among researchers for their huge structural diversity and have proved to be a valuable source for drug discovery. Among these products, a group of steroid antibiotic compounds represented by fusidic acid and helvolic acid exhibits remarkable antimicrobial activities by inhibiting the translational system. However, our lack of biosynthetic knowledge on steroidal natural products has prevented their production utilizing biosynthetic enzymes.
|
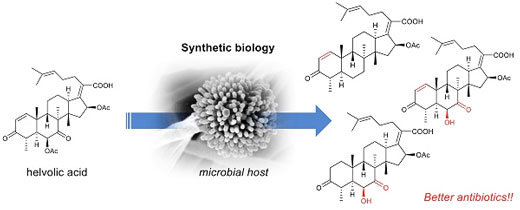 |
| Artificial biosynthesis of steroid antibiotics. A synthetic biology method enables us to create novel antibiotics in an efficient and easy way. (Image: Ikuro Abe)
|
|
In the current study (Nature Communications, "Biosynthesis of helvolic acid and identification of an unusual C-4-demethylation process distinct from sterol biosynthesis"), the research group led by postdoctoral researcher Dan Hu and Professor Ikuro Abe at the Graduate School of Pharmaceutical Sciences, the University of Tokyo, identified the biosynthetic enzymes of helvolic acids, and constructed a helvolic acid-producing system by expressing them in Aspergillus oryzae, a type of mold commonly known as koji, which is used for fermentation in food production.
|
|
The researchers isolated novel helvolic acid analogs by isolating the biosynthetic intermediates from the system. Remarkably, some of the analogs exhibited better activity than helvolic acid, thereby illustrating the utility of this production system.
|
|
By comparing the structures, the research group found that the structural difference at the A and B rings of the compounds are important for antibiotic activity. Furthermore, the group clarified the C-4 demethylation pathway, which involves a P-450 oxidase and a reductase.
|
|
"In this study, we developed a production system for steroid antibiotics," says Abe. He continues, "Our results could pave the way for the development of a synthetic biology method that can lead to the creation of novel molecular scaffolds for efficient and easy antibiotic drug synthesis."
|

