| Aug 16, 2018 |
Reconstructing skin on a chip
|
|
(Nanowerk News) Microfluidics could fulfill a growing need for alternatives to animal testing for the development of pharmaceuticals and cosmetics. A multidisciplinary team, led by Zhiping Wang from the A*STAR Singapore Institute of Manufacturing Technology, and Paul Bigliardi from the A*STAR Institute of Medical Biology, have produced a scalable credit-card sized device that simultaneously facilitates skin cell culture and testing (Materials Today, "Full-thickness human skin-on-chip with enhanced epidermal morphogenesis and barrier function").
|
|
State-of-the-art alternatives to animal testing rely on reconstructed skin. However, these three-dimensional tissue models are typically generated from static cell cultures on a collagen matrix that readily shrinks.
|
|
“When collagen contracts, we don't know whether compounds under investigation are going through the skin or through gaps between the device and the skin during permeation tests,” explains Gopu Sriram, one of the lead authors.
|
|
To address these problems, the researchers developed a method to grow skin on a matrix using the protein fibrin, preventing skin contraction. The skin is grown directly in the microfluidic device where the tests are conducted, without further manipulation or transfer.
|
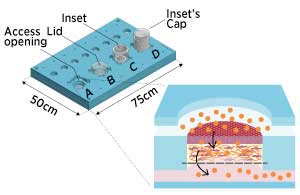 |
| Microfluidic skin-on-a-chip device working as an open system (A), a lidded bioreactor (B), and an in vitro analysis system fitted with an open (C) or a capped inset (D). The close-up shows a graphic representation of a functionality test on skin-on-chip equivalents under dynamic flow conditions. (Licensed under CC BY-NC-ND 4.0 © 2018 G. Sriram et al.)
|
|
Skin cultured in the microfluidic device exhibited enhanced maturation of the epidermis, the top protective layer of the skin. This translated to a nearly two-fold increase in epidermis thickness compared to standard skin equivalents.
|
|
“This enhanced epidermis correlated with lower chemical permeability than in conventional systems,” says Yuri Dancik, another lead author.
|
|
“Compared to conventional skin reconstruction, the skin-on-chip platform offers better skin morphology and performance, in terms of barrier function,” adds Wang. It can also facilitate downstream assays using commercially available skin equivalents or natural skin.
|
|
According to Massimo Alberti, another lead author, these enhancements stem from the use of microfluidics. Under static conditions, nutrients and medium passively diffuse through the skin. By contrast, in the microfluidic chip, a continuous flow generates pressure that pushes the culture medium through the matrix and may act as a “stressor for the cells and the extracellular matrix, which may also activate some mechanically-triggered signaling pathways,” he says.
|
|
This stimulation also promotes the formation of a superior basement membrane, a “Velcro-like protein layer that anchors the epidermis to the connective tissue called dermis,” says Sriram.
|
|
In addition to automating their system, the researchers are currently working to improve their model to better mimic natural human skin. They plan to increase the complexity of their model by adding immune cells and enhancing its barrier function.
|
|
They are also optimising the microfluidic device by simulating blood flow dynamics and implementing additional microenvironment controls “to promote conditions that will bring the system closer to human skin,” says Alberti.
|

