| Jan 31, 2024 |
Microfluidic vortex flows spur advances in robust cellular bioreactors
|
|
(Nanowerk News) Immobilizing fragile yet functional biomolecules within the microscopic confines of lab-on-a-chip devices has confounded researchers seeking to create efficient microfluidic platforms for diverse biochemical applications from biosensing to biocatalysis. The enduring challenge stems from biomaterials’ susceptibility to irreversible deformation when adhered using stationary methods ill-suited to their delicate conformations. However, recent advances in microengineering and self-assembly processes are shifting paradigms.
|
|
As detailed in research published in Advanced Functional Materials ("Flow-Induced Microfluidic Assembly for Advanced Biocatalysis Materials"), an interdisciplinary group led by Christof M. Niemeyer pioneered an integrated microfluidic system harnessing the power of microfluidic flows themselves to actively structure complex biological compounds. Their custom-built microreactor platform utilized fluidic vortex forces to gently adhere DNA, enzymes and even viable bacteria, while configuring the substances into functional films.
|
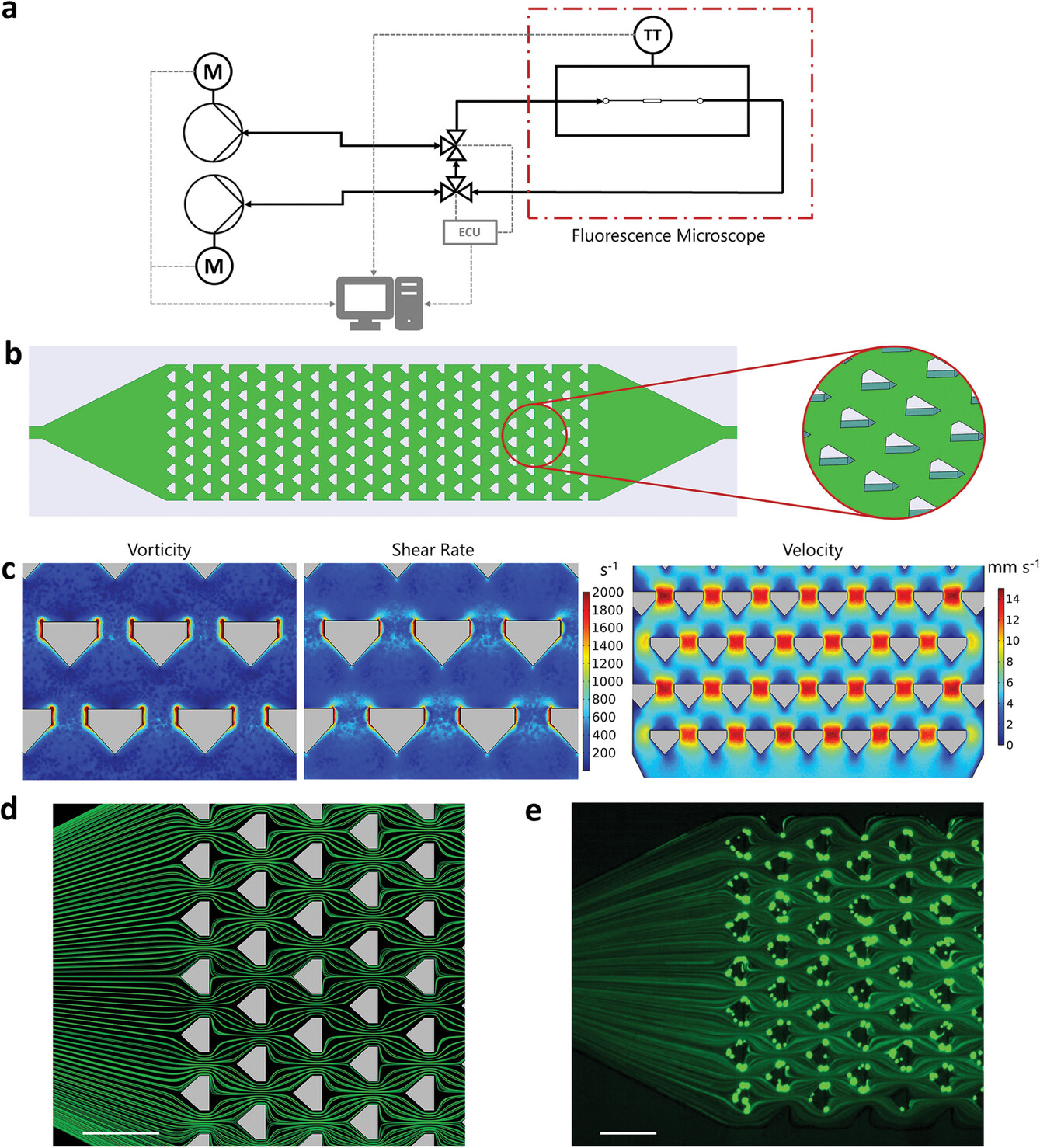 |
| Microfluidic platform for flow-induced deposition of various biomaterials. a) Schematic overview of the microfluidic setup. The microfluidic deposition reactor is positioned inside a microscope (dash-dotted line) for online measurements of the deposition process via brightfield as well as fluorescence images. The temperature transmitter (TT) allows automatic temperature control inside the microscope. Computer-controlled motorized (M) syringe pumps enable automatic flow rate changes using a custom written script. A (semi-)continuous flow without volume restrictions can be realized with two independently controllable pumps by means of a circuit with two valves that are automatically switched by an electrical control unit (ECU). b) Schematic illustration (top view) of the microfluidic deposition reactor (left) and a close-up (oblique view) of the pillars inside the reactor (right). The 90 μm wide pillars with a 60 μm gap in between lead to suitable fluidic conditions for the flow-driven immobilization of biomaterials. c) Results of vorticity, shear rate, and velocity simulations. Note that the scale of vorticity and shear rate simulation results only covers the range 0–2000 s−1 to make the smaller values visually distinguishable. This scaling results in all values >2000 s−1 appearing as dark red. d) Streamline simulation. e) Experimental validation using green fluorescent beads with a diameter of 1 μm. To visualize the streamlines, the fluorescence image was acquired with an exposure time of 250 ms. Scale bars: 200 μm. (Reprinted with permission by Wiley-VCH Verlag)
|
|
The automated setup further enabled exceptional experimental control, from programmable flow modulation to real-time microscopic visualization and integrated downstream analysis. These versatile capacities empowered systematic assessments uncovering key engineering principles for translating laboratory-based accomplishments into radically enhanced, industrially scalable biotechnologies.
|
|
The study’s insights moreover promise to illuminate fresh biomimetic approaches for designing next-generation adaptive, bio-instructive materials under non-equilibrium conditions. Thereby demonstrating microscale fluid manipulations’ potential to resolve longstanding immobilization barriers, while unveiling unconventional routes to bio-inspired discovery.
|
|
The researchers constructed a purpose-built microreactor containing an array of pillars, designed to generate microscale vortices and shear forces capable of capturing biomolecules from solution and adhering them to the reactor walls. Computational models guided geometrical optimization of pillar spacing and channel width to ensure suitable fluidic conditions for deposition across a 5 mm long, 1.1 mm wide chamber.
|
|
This microreactor was then integrated into an automated microfluidic system permitting regulated injection of samples, mixing with reagents, and programmable changes in flow velocity. The setup additionally enabled microscopy visualization and fluid composition analysis, granting researchers exceptional experimental control and characterization capabilities.
|
|
Initial trials focused on DNA hydrogels—highly hydrated polysaccharide networks formed through molecular entanglement of single stranded DNA amplified via rolling circle techniques. These porous matrices display utile biosensing and biocatalysis properties derived from facile integration of functional moieties onto complementary DNA base pair regions.
|
|
High shear microfluidic flows successfully deposited DNA rolling circle amplification products as filamentous hydrogels adhering at pillar edges across the 5mm long, 1.1mm wide custom microreactor. Consecutive static incubation enabled in-situ hybridization and chemoselective immobilization of a SpyTagged protein onto complementary SpyCatcher-labeled oligonucleotides previously hybridized to the DNA scaffold.
|
|
However, the condensed nature of these materials restricted penetrative diffusion. Steric congestion substantially limited maximum modification densities achievable through sequence-directed protein conjugation to just 5-9% of available DNA sites—deemed insufficient for viable biocatalytic applications.
|
|
Later assessments utilizing enzyme variants genetically fused to SpyTag/SpyCatcher domains verified direct shear-based deposition onto microreactor walls proved far more efficient. Unfortunately, the harsh hydraulically-induced forces disrupting protein tertiary structures to promote adhesion concurrently tended to irreversibly denature catalytic function—an intrinsic trade-off negating utility.
|
|
A revised two-stage technique retained efficacy of the shear-induced adhesion mechanism while safeguarding enzyme activity. An initial protein scaffold was forcibly deposited under harsh flow conditions before gentle perfusion allowed native counterparts to bind. This rescued approach substantially increased bioreactor productivity over single-step processes. But long-term robustness and stability was unattainable with the protein systems tested.
|
|
However, translating principles behind shear-based deposition to bacterial cells proved transformative. Subjecting cultures of E. coli displaying green fluorescent protein to analogous microfluidic flows readily immobilized substantial quantities as surface-adhered biofilms, with cells remaining viable. Enzyme-expressing variants similarly assembled into functional biocatalytic films.
|
|
These living cellular materials exhibited major advantages over isolated protein alternatives, with exceptional resilience against shear deformations arising from protective enzymatic sequestration inside cell walls and membranes. Product formation rates ranged from 7000-8000 grams per liter per day and endured undiminished for over 240 hours of continual operation – 60 to 140 times higher than previous microfluidic bioreactors.
|
|
Researchers suggest the self-regenerative properties of dividing cells additionally contributed to favorable microreactor lifetimes. Controlled experiments indicated firmly anchored cells repopulated vacant regions, while supplemental nutrient perfusion enabled population recovery following intentional starvation. Overall, the bacterial biofilm’s physical durability and biological vitality synergized for unmatched microfluidic bioprocess outputs.
|
|
With optimization, this pioneering flow-driven cell immobilization strategy may grant access to versatile, highly efficient microfluidic modules for diverse biotechnological and biochemical applications through scalable ‘numbering-up’ manufacture. The insights gained also illuminate fresh possibilities for designing dynamic living materials under non-equilibrium conditions.
|
|
Further efforts should concentrate on assessing long-term operational stability over months of continual usage, evaluating transferability to alternate enzymatic pathways, and systematically varying physical parameters to deduce universal engineering principles. Nevertheless, the impressive productivity returns already achieved underscore the transformative potential of actively utilizing fluid flows to structure functional biomaterials.
|

