Mastering the flow in tiny spaces – an overview of microfluidics
Contents
Introduction
Microfluidics is an interdisciplinary field that combines principles from physics, chemistry, and engineering to create devices that can process and analyze minuscule volumes of liquids. Often, microfluidic devices and systems are designed to replicate or model the behaviors observed in natural systems.
For example, microfluidic devices can be engineered to replicate the behavior of blood vessels or organs, such as the lungs or liver, to study disease mechanisms or drug interactions. Additionally, microfluidic devices have been developed to model ecological systems, such as the flow of water through soil or the transport of nutrients through plant tissues.
Think of it this way: The human body can be conceptualized as a macro-scale manifestation of a microfluidic systems. Our existence and physiological functions are intricately tied to this complex system of microfluidics, which allows our bodies to operate with remarkable efficiency and precision.
The circulatory system, which includes the heart, blood vessels, and capillaries, is a prime example of microfluidic behavior. Most capillaries are only about 8-10 micrometers in diameter, and they deliver blood, nutrients and oxygen to cells throughout the body. They are so small that red blood cells have to pass through in a single file line.
Blood flows through these tiny vessels in an organized and predictable manner, allowing it to deliver essential nutrients, oxygen, and signaling molecules to every cell in the body.
Microfluidics also plays a crucial role in the lymphatic system, which helps maintain the body's fluid balance and immune function. The lymphatic vessels transport lymph fluid, a fluid that contains immune cells and other substances, throughout the body. The movement of lymph through these vessels is facilitated by a series of one-way valves and muscle contractions, which create a microfluidic system that helps clear waste products and fight infections.
You probably interact with microfluidic devices quite frequently in your daily life.
For instance, inkjet printers use microfluidic technology to deposit tiny droplets of ink onto paper. The ink is pushed through a small nozzle by a tiny pump, and the droplets are precisely positioned to create text and images. In a similar manner, 3D printers utilize a microfluidic nozzle to release molten polymer or other liquid materials.
Fountain pens rely on microfluidic principles to facilitate ink flow through their tips and onto paper. They use capillary action, which is a fundamental principle of microfluidics, to draw ink from the reservoir into the nib of the pen. The nib of a fountain pen is made up of a series of tiny capillaries, or channels, that wick the ink from the reservoir and transport it to the paper. When the nib comes into contact with the paper, the ink flows out of the capillaries and onto the surface. The thickness and wetness of the line can be controlled by the width of the nib and the pressure applied to it.
Nebulizers designed for asthma patients disperse a mist of tiny drug droplets via microfluidic channels. The nebulizer works by using a small pump to force liquid medication through a series of microscale channels or nozzles, which break up the liquid into droplets. By controlling the flow of fluid through these channels, nebulizers can control the size and distribution of the droplets that are produced and optimize the delivery of medication to the lungs.
And let's not forget how microfluidic test strips for blood glucose enable those living with diabetes to make life-sustaining decisions while maintaining their independence and active lifestyles:
What is microfluidics?
Microfluidics encompasses both the technology of creating tiny devices that control the flow and confinement of fluids and the science of fluid behavior in fluidic systems with dimensions ranging from a few micrometers to a millimeter. In microfluidics, fluids are dealt with in extremely small volumes, reaching down to femtoliters (fL, a quadrillionth of a liter, 10-15).
Fluids can be moved in multiple ways: via a mechanical pump, utilizing the surface charges of specific materials, or through capillary action, which is also known as wicking – which involves the movement of liquids through narrow spaces, propelled by the energy stored within the liquid.
Liquids exhibit peculiar behavior at small scales, different from what is observed in everyday life because of the unique physical and chemical properties that emerge at this scale. Unlike the turbulent and erratic flow patterns of garden hoses and showerheads, in microchannels, where space is confined, flows tend to be surprisingly steady. This phenomenon, known as laminar flow, is one of the most remarkable aspects of microfluidic systems. In laminar flow, fluids flow along the channel in organized, parallel streams, and the movement of fluids and particles can be precisely predicted through mathematical models. This property of laminar flow is crucial for applications that require high levels of precision, such as medical device design and engineering.
These unique characteristics that open up new avenues for scientific exploration and innovation. Some of the key differences are:
Surface tension: At the microscale, the effects of surface tension become much more pronounced, leading to the formation of droplets and the manipulation of fluids in ways that are not possible at the macroscale.
Diffusion: The rate of diffusion – which arises from random movement of particles – is much higher at the microscale, which means that the mixing of fluids and the transport of molecules occur much more quickly.
Reynolds number: The Reynolds number, which determines the flow behavior of a fluid, is much smaller at the microscale. This means that fluids exhibit laminar (smooth) flow instead of turbulent flow, which is observed at the macroscale.
Viscosity: The viscosity of fluids is much higher at the microscale, which makes it possible to control the flow of fluids and perform precise manipulations.
Capillary forces: At the microscale, capillary forces become much stronger, which can be used to control the movement of fluids and to create new types of fluidic systems.
History of microfluidics
The field of microfluidics was first introduced in the late 20th century, and since then, it has made significant contributions to many areas of research and technology, including biotechnology, nanotechnology, and analytical chemistry.
A seminal paper in 1979 by researchers at Stanford University is widely regarded as the birth of microfluidics as a field of its own. In this paper, the researchers describe how they created a device using photolithography and etching techniques. It has an injection valve and a 1.5 m long capillary coil. They also made a thermal conductivity sensor using techniques developed in the semiconductor industry and attached it to the end of the capillary as a detector. When the detector is combined with the capillary to separate the gases in the system, it can accurately analyze the composition of the injected gas mixture with high sensitivity and specificity.
This device is now widely regarded as the first Lab-on-a-Chip although this term had not been coined at this point.
Researchers now realized that microfluidics had the potential to enable the development of new, more powerful techniques for molecular analysis. In light of this, they began creating devices that could manipulate fluids on a small scale. They focused on creating pumps and valves that could precisely control and manipulate fluid flow, allowing for the development of reliable microfluidic devices.
In 1984, Charles Hull invented stereolithography (SLA), a 3D printing technology that revolutionized the microfluidics industry (he coined this term in his 1984 patent application for "Apparatus for production of three-dimensional objects by stereolithography" (pdf) U.S. patent US4575330). SLA creates three-dimensional objects by stacking two-dimensional laminae. Software breaks down a 3D model into a sequence of 2D layers that are projected in series onto a build platform submerged in UV curable resin. After each layer solidifies, the platform moves upwards, and the next layer is cured. This process repeats until the full object is manufactured. Researchers could now create custom parts for short production runs without needing expensive or specialized equipment and tooling.
Then, in 1990, Manz e.al. suggested that microscale fluidic devices could be used to create a complete analysis system – a "Total Analysis System" (TAS) that could automatically perform all the required analysis functions, including sampling, sample transport, preparation, chemical reactions, separations, and detection. If the system had microscale dimensions, it would be called a miniaturized Total Analysis System (µTAS). The researchers explained that the benefits of fluid mechanics on a small scale would result in faster and more efficient analysis as many channels could be fabricated into a small area to enable simultaneous analysis of multiple samples.
This paper showcased the potential of microfluidic devices and laid the foundation for many advancements in the field.
How microfluidic devices are fabricated
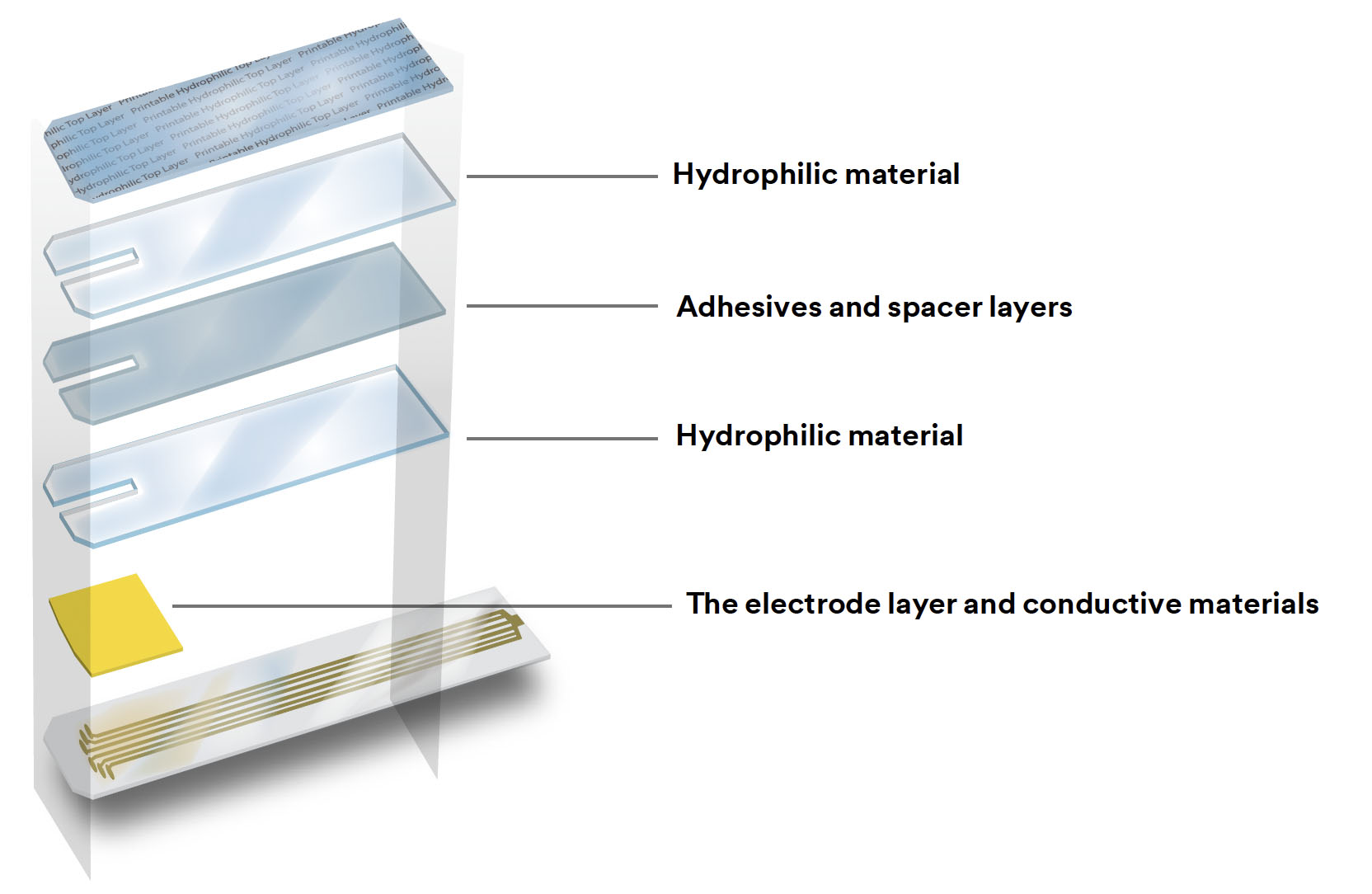
Microfluidic devices consist of microscale channels, chambers and valves that allow the precise control of fluid flow, mixing, and analysis. These devices are designed and manufactured using various materials and fabrication techniques and benefited from the advances in micromanufacturing techniques.
Microfluidic devices are typically fabricated using various techniques, including:
Photolithography: This is a process in which a pattern is created on a photosensitive material using light and chemicals. The pattern is then transferred to a substrate material to create the microfluidic channels and chambers.
Soft Lithography: This is a technique that uses soft materials, such as elastomers or polymers, to create patterns that can be transferred to a substrate material. Soft lithography is a low-cost and simple method for fabricating microfluidic devices. Advanced applications of this technique have led to shape-programmable 3D microfluidics, which are assembled from a bilayer of channel-embedded polydimethylsiloxane (PDMS) and shape-memory polymers via compressive buckling.
Micromachining: This is a technique that uses mechanical or laser processing to create the microfluidic channels and chambers in a substrate material. Micromachining is often used for creating high-precision microfluidic devices.
3D Printing: This is a process in which a 3D object is created by layering materials, such as polymers or ceramics, using a specialized printer. 3D printing is a rapidly growing area in microfluidics, and it allows for the rapid prototyping and customization of microfluidic devices. For instance, researchers demonstrated 3D printing microfluidic channels on a curved surface, providing the initial step for someday printing them directly on the skin for real-time sensing of bodily fluids.

Molding: This is a process in which a mold is created and then used to produce a large number of microfluidic devices. Molding is a low-cost and efficient method for mass-producing microfluidic devices.
The choice of fabrication technique depends on the specific requirements of the microfluidic device, such as size, precision, and material compatibility. Additionally, the cost and complexity of the fabrication process are important factors to consider when selecting a fabrication technique.
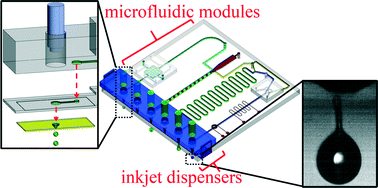
Microfluidic devices fabricated using lithography-based fabrication processes are fairly expensive. To reduce costs in developing and producing microfluidic devices, researchers started using paper, for instance to inkjet print active microfluidic chips on normal paper. Paper is advantageous because it allows fluid to be transported by capillary action, eliminating the need for power-requiring pumps. This technique has been used in successful commercial devices such as pregnancy tests, pH strips, and glucose sensors. In 2007, the Whitesides Group demonstrated how functionalized chromatography paper could be used for quick, multiplex analysis, leading to the rise of paper analytical devices (PADs) in the microfluidics community.
In a recent game-changing approach for biotechnology research, engineers are developing a transformative way to fabricate microfluidic devices, where solid walls are replaced by transparent, morphing fluid walls using only biocompatible materials. This innovative protocol offers significant benefits to scientists in biology and biomedicine who can now easily create their own cellular microfluidic environments in minutes – almost as quickly as the circuit pattern can be drawn on paper – using truly cell-friendly materials, standard Petri dishes and culture media.
Fluids find path through a maze with a single inlet and outlet. The circuit was built by jetting FC40 through media plus red dye; when blue dye is pumped into the inlet, it takes the path of least resistance through the maze. (Video: Oxford Thermofluids Institute)
Microfluidics applications
A myriad of applications has been developed over the years that we can’t all cover here in this article. So, we are highlighting just a few examples.
Arguably one of the most promising opportunities of microfluidics for diagnostics reside in point-of-care (POC) applications – i.e., in medical testing at or near the site of patient care – because a number of unmet needs can be fulfilled by microfluidic devices due to their portability, short sample processing time, and flexibility. In these applications, small volumes of fluid can be processed and analyzed much faster than traditional laboratory techniques, leading to faster and more accurate results.
For instance, researchers have developed a new microfluidic chip for diagnosing diseases that uses a minimal number of components and can be powered wirelessly by a smartphone. This innovation opens the door for faster and more affordable at-home medical testing.
The key benefit of microfluidics is the ability to handle extremely small volumes of fluid, which makes it ideal for applications in biotechnology that require high sensitivity and precision such as cell analysis, protein purification, and DNA amplification. For instance, researchers have demonstrated a glass microchip for ultrafast separation and purification of DNA fragments. The device uses only tiny amounts of DNA, like in medical diagnostics or in forensics, and is capable of fractionating DNA fragments within just a few minutes, while conventional approaches take hours.
Another application is in drug discovery and development where microfluidic devices can be used to screen large numbers of drug candidates in a short amount of time, leading to the rapid identification of potential new drugs. Additionally, by simulating complex physiological environments, researchers can study the interactions between drugs and biological systems.
Point-of-care diagnostic devices, such as rapid blood glucose meters, use microfluidic technology to analyze small samples of fluid, such as blood or saliva, for the presence of various biomarkers. These devices are portable, easy to use, and provide quick and accurate results, making them a valuable tool in medical diagnosis and treatment.
Novel microfluidics techniques that have emerged include droplet microfluidics – involving the encapsulation of a reaction in the discrete compartments of an emulsion – which led to the development of this technique for high throughput PCR, enzyme screening, single cell antibody screening and the controlled manufacture of gold nanoparticles.
Microfluidic chips are particularly promising in cancer diagnosis. In most cases, a tissue biopsy is the initial means of making a diagnosis. With increasing accuracy, liquid biopsies – where circulating tumor cells (CTCs) are isolated from blood samples – are becoming a viable complement or even alternative to invasive biopsies of metastatic tumors. In one example, for instance, rather than using magnetic and microfluidic methods that are used in isolating CTCs, researchers used a static isolation in the form of micro-arrays of single-walled carbon nanotubes.
Rare tumor cells can be physically captured from blood using microfluidic traps developed by various research groups (there is an excellent overview article here: “Tumor-on-a-chip: from bioinspired design to biomedical application”. In addition, microfluidic genetic chips have enabled rapid sequencing of the human genome and the creation of personalized DNA tests, such as 23andMe. Microfluidics has been a crucial technology in making these advancements possible.
Microfluidics'role in nanotechnology
Nanomaterials play a significant role in microfluidics research by enabling precise control of fluid flow, enhancing detection sensitivity, and enabling the fabrication of functional devices. Nanotechnology benefits from microfluidics in several areas:
Synthesis: Microfluidic reactors are used to synthesize nanomaterials such as nanohybrids and macroscopic graphene fibers.

During the recent development of mRNA vaccines for the Covid-19 epidemic, massive quantities of mRNA sequences had to be synthesized and packaged into the lipid nanoparticles that deliver them into cells. Advanced high-throughput microfluidic devices were used in this effort.
Analysis: Researchers have demonstrated high-throughput, label-free nanoparticle analyzers that can be used for the rapid (seconds) characterization of a complex mixture of nanoparticles of different sizes, and to detect bacterial viruses in both salt solution and in blood plasma.
Nanomaterial Applications: Heat has become one of the most critical issues in computer and semiconductor design. To improve on the currently used heat sinks, researchers have demonstrated a microfluidic technique of using thermally conductive and magnetic nanoparticles that can form low-dimensional fins in the vicinity of hot spots.
In another example, nanoparticles can be used as labels for detecting biomolecules as well as material to create functional coatings for microfluidic surfaces, improving device performance and reducing nonspecific binding.
More recently, microfluidics researchers have used graphene, which has unique mechanical, electrical, and optical properties that make it attractive for microfluidic applications. Additionally, the high surface area of graphene and its derivatives can be utilized for enhanced capture and separation of biomolecules.
For instance, researchers have fashioned a thin film of graphene and a support layer of PMMA for use as a diffusion barrier in a microfluidic chip. Another group has demonstrated that an array of graphene transistors integrated with microfluidics can be employed to detect the change to the cell surface charge of a cell infected with a virus.
The future: Lab-on-a-chip and organ-on-a-chip
The terms microfluidic chip and lab-on-a-chip (LOC) are often used interchangeably, but they can refer to slightly different things.
A microfluidic chip refers to a small device that contains microscale fluidic channels and chambers, which are used for manipulating and analyzing small volumes of fluids. A microfluidic chip can be used for a variety of purposes, including chemical synthesis, biological analysis, and drug discovery.
Lab-on-a-chip, on the other hand, refers to a type of microfluidic chip that is designed for a specific analytical task, such as diagnosing a disease or determining the presence of a specific chemical in a sample. A LOC typically integrates several different analytical techniques, such as separation, detection, and analysis, into a single compact device.
Lab-on-a-chip technology is a captivating field of study that offers the prospect of using portable monitoring devices to examine patient samples at the bedside or monitor water quality in the field. Nonetheless, attaining a high level of sensitivity to the concentration of targeted analytes of interest can be challenging, particularly when working with extremely small volumes of liquid.
In the ever-evolving landscape of microfluidic systems for biomedical research, the latest – and highly promising – developments are organs-on-a-chip.
The use of engineered tissues has emerged as a vital tool for disease modeling and drug testing in a human context. However, researchers face a significant hurdle in creating a physiological communication system between multiple engineered tissues that can replicate the complex functions of the human body and its systemic diseases. To address these issues, researchers are developing various models of organ-on-chip devices.
An organ-on-chip device is a microfluidic system that mimics the structure and function of human organs, such as the lungs, liver, heart, or kidney, at a small scale. The device consists of a transparent, flexible chip with channels that contain cells, which are grown to mimic the behavior and physiology of the cells in the targeted human organ.
These chips, which are typically the size of a computer memory stick, can simulate different aspects of organ function, such as blood flow, breathing, or fluid exchange, by controlling the micro-environment surrounding the cells. By replicating the microstructure and function of human organs, these devices can be used to test the efficacy and safety of drugs and to study disease mechanisms in a controlled environment that closely mimics the human body.
Organ-on-chip technology offers a promising alternative to traditional animal and in vitro models that are often limited in their ability to accurately predict how drugs will behave in humans. With the ability to precisely control the environment surrounding the cells, organ-on-chip devices allow for a more accurate and realistic testing environment, which could reduce the cost and time required for drug development and ultimately lead to more effective treatments.
For example, researchers have developed a multi-organ chip consisting of engineered human heart, bone, liver, and skin that are linked by vascular flow with circulating immune cells, to allow recapitulation of interdependent organ functions.
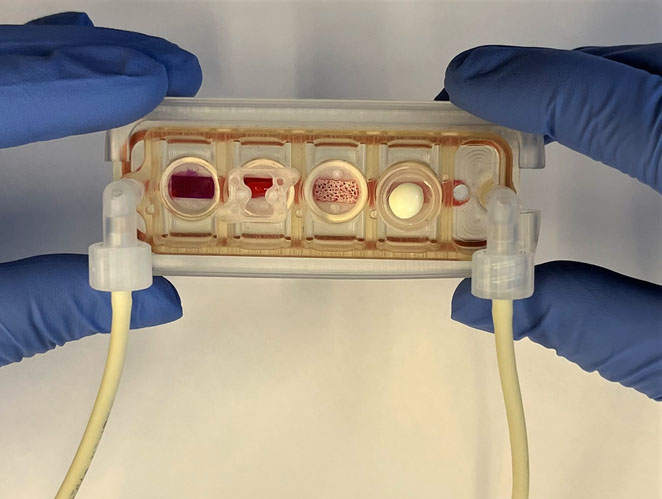
Organ-on-chip devices are also used as a tool to help scientists tease out the immune system’s mysteries. In addition to allowing researchers to probe the normal function of the immune system, these chips can also be used to predict immune responses to various vaccines and help select the best performers, offering significant improvement over existing preclinical models like cells in a dish and non-human primates.
Yet another leading-edge example of organ-on-chips are artificial placentas that are produced with customized hydrogel membranes directly within microfluidic chips, which are then populated with placenta cells. This makes it now possible to provide clarity in some vital research issues, such as the exchange of glucose between mother and child.
As Albert Folch, Professor of Bioengineering, University of Washington, wrote: “Imagine going to the doctor, getting a biopsy extracted, and in less than a week, by using our microfluidic [organ-on-chip] device, the doctor can figure out which drug cocktail works best to remove your tumor. That is still in the future, but what we do know is that the future will be microfluidic.”
Frequently Asked Questions (FAQs) about microfluidics
What is Microfluidics?
Microfluidics is the science and technology of systems that process or manipulate small amounts of fluids, using channels with dimensions of tens to hundreds of micrometers.
Why is Microfluidics important?
Microfluidics is important due to its range of potential applications in various fields such as biology, chemistry, and medicine, among others. It's used in DNA chips, lab-on-a-chip technology, micro-propulsion, and micro-thermal technologies.
What are the benefits of using Microfluidics?
Benefits of microfluidics include the ability to perform analyses more quickly and efficiently than traditional methods, increased control over experimental conditions, and the ability to work with very small sample sizes.
What materials are used in Microfluidics?
Common materials used in microfluidics include glass, silicon, and various types of polymers. The choice of material depends on the specific application and the type of analysis being performed.
How is Microfluidics applied in healthcare?
In healthcare, microfluidics can be used for point-of-care diagnostic tools, drug discovery and delivery systems, and in the creation of artificial organs. It has been especially influential in the development of portable diagnostic devices.
How are Microfluidic devices fabricated?
Microfluidic devices are usually fabricated using photolithography, soft lithography, and other microfabrication techniques. These processes allow for precise control over the dimensions and geometry of the channels and features in the device.
What is a Lab-on-a-Chip device?
A Lab-on-a-Chip device is a miniaturized device that integrates several laboratory functions onto a single chip only millimeters to a few square centimeters in size. These devices utilize microfluidic technology to handle small fluid volumes.
What is Digital Microfluidics?
Digital Microfluidics is a sub-field of microfluidics which allows for the manipulation of discrete micro-droplets, independently of each other. It's often used in applications where high throughput or precise control over individual droplets is required.
How does Microfluidics relate to Nanotechnology?
Microfluidics often works together with nanotechnology. Microfluidics provides a way to handle and process small volumes of fluids, which can be used to create, manipulate, or analyze nanoscale particles or molecules.
What are Microfluidic Chips?
Microfluidic chips are devices that use small fluid volumes. The chips have micro-channels etched into them, which allow for fluid to flow through. These devices are used in a wide variety of applications, from medical diagnostics to chemical synthesis.
What fields commonly use Microfluidics?
Microfluidics is commonly used in fields such as biotechnology, chemistry, physics, and materials science, among others. It's particularly popular in fields that require precise control over small volumes of fluids.
What are some challenges in Microfluidics?
Some challenges in microfluidics include issues with fabrication, including the need for high precision and cleanliness, issues with fluid handling, including the effects of surface tension and evaporation, and issues with integrating these devices into larger systems.
How is Microfluidics transforming diagnostics?
Microfluidics is transforming diagnostics by enabling the development of point-of-care devices that can perform complex tests rapidly, with less sample volume, and at a lower cost than traditional laboratory methods.
How does Microfluidics enhance drug delivery?
Microfluidics enhances drug delivery by allowing for precise control over drug formulation and release. This can lead to more efficient and targeted delivery of therapeutics, reducing side effects and improving patient outcomes.
What is the future of Microfluidics?
The future of microfluidics is likely to see even more integration with other technologies like nanotechnology and biotechnology, further miniaturization and increased automation, and wider adoption in various industries, especially healthcare and environmental monitoring.
