| Aug 30, 2021 | |
Hydrogels |
|
|
Contents
|
|
| (Nanowerk Spotlight) Hydrogels are fascinating natural or synthetic polymer materials that exhibit very versatile chemistries and physical or biological properties. They are 3D networks of either physically or chemically crosslinked polymers that resemble organic tissues and that can hold large amounts of water within their interlocked molecular network. | |
| These soft, translucent materials swell or shrink in response to water absorption or desorption and can hold a large amount of water without losing their structural integrity. | |
| Hydrogels were first proposed for biological use in 1960. In order for a material to be considered a hydrogel, water must constitute at least 10% of its total weight or volume. | |
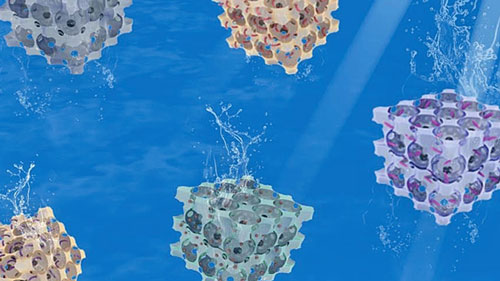 |
|
| Illustration of 3-dimensional macroporous hydrogels. (Image: Carnegie Mellon University) | |
| The ability of hydrogels to absorb water arises from hydrophilic functional groups attached to the polymeric backbone, while their resistance to dissolution arises from cross-links between network chains. Many materials, both naturally occurring and synthetic, fit the definition of hydrogels. | |
| Natural hydrogels include collagen, silk fibroin, hyaluronic acid, chitosan, alginate and hydrogels derived from decellularized tissues. Their unique properties include: biocompatibility, biodegradability, low cytotoxicity, the possibility to tailor the hydrogel into an injectable gel and their similarity to physiological environment. | |
| However, natural hydrogels do have some limitations, for example they do not have strong mechanical properties and are not easily controllable due to their batch-to-batch variation. For these reasons, natural hydrogels are often combined to synthetic ones, creating composite polymers. | |
| Hydrogels have become a highly relevant biochemical scaffold due to their tunable properties, inherent biocompatibility, and similarity with tissue and cell environments. Over the past decade, hydrogels have developed from static materials to “smart” responsive materials adapting to various stimuli, such as pH, temperature, chemical, electrical, or light. | |
| Besides biomedical applications, this makes them useful materials in soft robotics as well as responsive and programmable materials that can be used for catalysts, chemical detectors, and absorbents for carbon capture. | |
Types of hydrogels |
|
| Hydrogels can be physical, chemical, or biochemical. Physical gels can undergo a transition from liquid to a gel in response to a change in environmental conditions such as temperature, ionic concentration, pH, or other conditions such as mixing of two components. Chemical gels use covalent bonding that introduces mechanical integrity and degradation resistance. Biochemical hydrogels contain biological agents like enzymes or amino acids that participate in the gelation process. | |
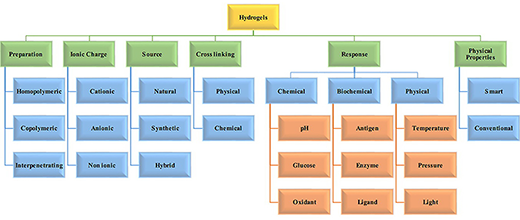 |
|
| Classification of hydrogels based on the different properties. (Source: doi 10.5772/64301) (click on image to enlarge) | |
How to make hydrogels |
|
| The three integral parts of hydrogel preparation are monomer, initiator, and cross-linker. | |
| In general, hydrogels can be prepared from either synthetic polymers or natural polymers. These polymers can be cross-linked to form hydrogels in a number of ways: Linking polymer chains via chemical reaction; using ionizing radiation to generate main-chain free radicals which can recombine as cross-link junctions; and physical interactions such as entanglements, electrostatics, and crystallite formation. | |
| Based on the methods of preparation, hydrogels may be classified as homopolymer, copolymer, semi-interpenetrating network (semi-IPN) and interpenetrating network (IPN). | |
| Homopolymeric hydrogels are referred to polymer network derived from a single species of monomer, which is a basic structural unit comprising of any polymer network. Copolymeric hydrogels are comprised of two or more different monomer species with at least one hydrophilic component, arranged in a random, block or alternating configuration along the chain of the polymer network. Multipolymer interpenetrating polymeric hydrogel (IPN), an important class of hydrogels, is made of two independent cross-linked synthetic and/or natural polymer component, contained in a network form. In semi-IPN hydrogel, one component is a cross-linked polymer and other component is a non-cross-linked polymer. | |
Hydrogel applications |
|
|
Hydrogels are used in many fields due to their specific structures and compatibility with different conditions of use. The flexibility of hydrogels makes it possible to use them in different conditions and their biocompatibility extends their applications to the medical sciences.
|
|
Drug delivery |
|
| Due to their compatibility with living tissues and ability to preserve embedded proteins in their natural state, hydrogels are good vehicles for delivering drugs into the body. | |
| Hydrogels as drug delivery agents are appealing for a number of reasons. They are mainly composed of water so they can shrink in their dry state to become small enough to swallow, and then can expand and swell in the stomach to avoid passing into the small intestine. They can also be loaded with medicine and release it in a controlled manner. | |
| Such controlled drug delivery systems are used to deliver drugs at certain rates for predefined periods of time. These systems have been used to overcome the limitations of regular drug formulations. | |
| There are various methods to prepare hydrogels for drug delivery applications. For instance, polymer polyethylene glycol can be used to incorporate micrometer-sized domains within the gel. These domains act as reservoirs for a protein drug when the drug molecules are also linked to polyethylene glycol. The chemical interactions between the modified protein and the reservoir domains allow a slow and steady release of the protein. | |
Tissue engineering |
|
| The ultimate goal of tissue engineering as a medical treatment is to replace or restore the anatomic structure and function of damaged, injured, or missing tissue – ultimately resulting in the ability to replace entire organs. At the core of tissue engineering is the construction of three-dimensional scaffolds out of biomaterials to provide mechanical support and guide cell growth into the new tissues or organs. | |
| Hydrogels are attractive for tissue engineering since their physical (i.e., mechanical strength and biodegradability) and biological properties (i.e., the biocompatibility and resemblance to the natural extracellular matrix) can be tailored to mimic tissues. By encapsulating cells of interest within microgels, biomedical engineers can tailor the desired approach for engineered tissue formation ranging from blood vessels to organs and bones. Injectable hydrogel can also provide a biomimetic scaffold for spinal cord regeneration. | |
| A new generation of adhesive hydrogels is used as wound dressing in surgical procedures to seal small wounds out of which air and body fluids could leak. The hydrogel polymers bond to biological tissues via three mechanisms - electrostatic attraction to negatively charged cell surfaces, covalent bonds between neighboring atoms, and physical interpenetration - making the adhesive extremely strong. | |
Environmental remediation |
|
| Heavy metal and dye pollution is commonly found in wastewater of many industrial processes and has been known to cause severe threats to the public health and ecological systems. | |
| Researchers have developed an eco-friendly way to remove heavy metals, dyes and other pollutants from water. The answer lies in filtering wastewater with a hydrogel material taken from plant cellulose and spiked with small carbon dots produced in a microwave oven. | |
 |
|
| An aqueous solution with methylene blue before and after being treated with lignocellulose hydrogel. (Image: Giuseppe Melilli) | |
Contact lenses |
|
| Synthetic hydrogels, mostly made from silicone, are being used commercially to make contact lenses; they are easy to work with and cost effective. Hydrogel materials used as a contact lens need to possess a great amount of water content, good mechanical properties, permeability toward oxygen, wettability of surface, good optical facilities, stability toward hydrolysis and sterilization, be nontoxic, and have enough biological tolerance for living cells. | |
Sensors |
|
| pH sensors. A prime example of how hydrogels are used as sensors is a pH sensor. A hydrogel is formed into a series of raised stripes called a ‘diffraction grating’, which is coated with gold on both the stripe surfaces and the spaces in between. The stripes expand and contract depending on the pH level of the environment. | |
| The sensor works by analyzing laser light reflecting off the gold coatings. Reflections from the stripes and spaces in between interfere with each other, creating a "diffraction pattern" that differs depending on the height of the stripes. | |
| These diffraction patterns indicate minute changes in the movement of the hydrogel stripes in response to the environment, in effect measuring changes in pH. | |
|
Radiation sensors. Another example is a plasmonic nanosensor hydrogel to detect therapeutic levels of radiation. The hydrogel matrix is infused with gold nanoparticles, which change their color upon exposure to radiation (visible to the naked eye). The intensity of the color increases with increasing radiation. These hydrogels can be used to report the dose of radiation delivered to different locations in the exposed area.
|
|
| Pollution sensors. Many strains of engineered bacteria can be used as sensors to detect environmental contaminants such as heavy metals. If deployed in the natural environment, these sensors could help scientists track how pollutant levels change over time, over a wide geographic area. In order to make this kind of deployment safe and prevent the bacteria from escaping into the environment and potentially spreading modified genes to other organisms, the bacterial sensors are encased in a tough hydrogel shell. | |
Functional hydrogels with advanced properties |
|
| Hydrogels that are electrically conductive allow additional fields of application, for example in the transmission of electrical signals in the body For instance, conductive hydrogels could be used to control the release of active drug substances in order to treat certain diseases locally in a more targeted manner. | |
| 3D-printable bio-activated nanocellulose-alginate hydrogel offers a platform for the development of hydrogel-based 3D bioprinting, wearable sensors, and drug-releasing materials for wound healing. | |
| Hydrogel electrolytes render supercapacitors extraordinarily stretchable and compressible. In combination with carbon nanotube composite paper electrodes, this hydrogel forms a supercapacitor that can be stretched to 1,000 percent in length and compressed to 50 percent in thickness with even gaining, not losing capacity. | |
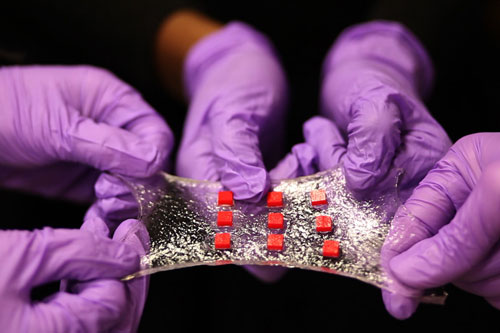 |
|
| A stretchy hydrogel can be embedded with various electronics. Here, a sheet of hydrogel is bonded to a matrix of polymer islands (red) that can encapsulate electronic components such as semiconductor chips, LED lights, and temperature sensors. (Image: MIT) | |
Hydrogels in soft robotics |
|
| Actuators | |
| Hydrogel materials possess intrinsic softness and they also exhibit other favorable properties that make them a perfect fabrication material for biomimetic soft robots: stretchability, biocompatibility, permeability, and stimuli-adaptability. | |
| But while hydrogels have garnered much interest in applications such as drug delivery or scaffolds for tissue growth, researchers have found it challenging to turn them into controllable actuators that simulate muscle movement. | |
| Most hydrogels swell or shrink in response to water absorption or desorption, but these deformations occur uniformly, whereas actual muscles deliver forces in specific directions. Furthermore, expelling and absorbing water from hydrogels is usually a slow process, and thus cannot match the fast responses of muscles. | |
| One research team was inspired by this unique behavior of sea cucumbers, that can freely change shape by reacting with water, to develop a water-driven self-operating soft actuator that exceeds the strength and speed of conventional soft actuators. | |
 |
|
| A programmable powerful and ultra-fast water-driven soft actuator inspired by the mutable collagenous tissue of the sea cucumber. (Image: POSTECH) | |
| Another team of researchers have designed a hydrogel whose properties differ considerably from those of other hydrogels. Their thermo-responsive hydrogel material can lengthen in one direction and contract in another without taking up or releasing water, allowing this muscle-like device to operate rapidly in an open-air environment. The secret to this behavior lies in the tiny nanosheets embedded in the hydrogel that harness electrostatic repulsion to make the hydrogel perform like a coiled spring. | |
| Inspired by the way plants grow toward light sources, a phenomenon known as phototropism, bioengineers have created a hydrogel that could be manipulated by light: | |
| Artificial skin | |
| By developing a unique silver-hydrogel composite that has high electrical conductivity and is capable of delivering direct current while maintaining soft compliance and deformability. | |
| To do this, they suspended micrometer-sized silver flakes in a polyacrylamide-alginate hydrogel matrix. After going through a partial dehydration process, the flakes formed percolating networks that were electrically conductive and robust to mechanical deformations. By manipulating this dehydration and hydration process, the flakes can be made to stick together or break apart, forming reversible electrical connections. | |
| The silver-hydrogel composite can be printed by standard methods like stencil lithography, similar to screen printing. The researchers used this technique to develop skin-mounted electrodes for neuromuscular electrical stimulation. The composite could cover a large area of the human body, like a second layer of nervous tissue over your skin. | |
| Self-healing hydrogels | |
| Self-healing gels are particularly of interest for conductive hydrogels in electrical or electronics applications. Supramolecular hydrogels are defined as gels that utilize noncovalent interactions to form three-dimensional entangled networks in a given solvent. | |
| For instance, researchers have developed a hybrid gel that is composed of conductive polymer and a metal-ligand supramolecule; the novel gel exhibits attractive properties associated with both conventional polymers, such as ease of synthesis and processing, and great self-healing performance at room temperature without any stimuli. | |
| In another example, researchers have designed an electronics-free, entirely soft robot shaped like a dragonfly that can skim across water and react to environmental conditions such as pH, temperature or the presence of oil. | |
| This reaction is based on hydrogels. By painting one set of wings with the hydrogel, the researchers were able to make their bot responsive to changes in the surrounding water’s pH. If the water becomes acidic, one side’s front wing fuses with the back wing. Instead of traveling in a straight line as instructed, the imbalance causes the robot to spin in a circle. | |
| Once the pH returns to a normal level, the hydrogel heals, the fused wings separate, and the bot once again becomes fully responsive to commands. | |
Magnetic hydrogels - ferrogels |
|
| Researchers are preparing magnetic-field-sensitive gels, called ferrogels, by encapsulating magnetite micro- or nanoparticles into chemically crosslinked hydrogels. By applying magnetic fields, the shape and behavior of the gels can be manipulated. | |
| For instance, scientists demonstrated the ability to switch the drug release profile of magnetic hydrogels between 'on' and 'off' mode. | |
| Using a magnetic field and hydrogels, researchers have also demonstrated a new possible way to rebuild complex body tissues, which could result in more lasting fixes to common injuries, such as cartilage degeneration. | |
| They found that if they added a magnetic liquid to a three-dimensional hydrogel solution, cells, and other non-magnetic objects including drug delivery microcapsules, could be arranged into specific patterns that mimicked natural tissue through the use of an external magnetic field. | |
Smart hydrogels |
|
| Materials are considered ‘smart’ when they can react on their own to external stimuli such as pH, temperature, light intensity, the presence of certain chemicals, radiation, etc. In the case of hydrogels, such stimuli-responsive hydrogels are able to dramatically change their volume and other properties in response to environmental stimuli. The result could be a release of their payload or a change in color. | |
| Smart hydrogel materials hold great promise for developing responsive materials that can be used for catalysts, chemical detectors, tissue engineering scaffolds and absorbents for carbon capture. | |
| One example is a hydrogel that releases drug molecules for pain management and tissue repair osteoarthritis patients. Only when a certain amount of pressure is applied to the joint, does the hydrogel release its anti-inflammatory medicine. | |
| Another example is a hydrogel that changes shape over time when temperatures change: | |
Frequently Asked Questions (FAQs) about hydrogelsWhat are hydrogels?Hydrogels are networks of polymer chains that are hydrophilic, often found as a colloidal gel in which water is the dispersion medium. They are highly absorbent (they can contain over 90% water) and are used in a variety of products and medical applications.
What are the uses of hydrogels?Hydrogels have many applications, including in agriculture as a soil conditioner, in healthcare for wound dressings, drug delivery and tissue engineering, in personal care products like baby diapers and contact lenses, and in food as additives.
What materials are used to create hydrogels?Hydrogels can be synthesized from a variety of materials, including synthetic polymers like polyvinyl alcohol, polyethylene oxide, and acrylates, as well as natural polymers like gelatin, collagen, and cellulose.
What properties do hydrogels have?Hydrogels are unique due to their high water content, softness, and ability to swell in water without dissolving. They can also respond to stimuli such as temperature, pH, and light, which make them suitable for many advanced applications.
How are hydrogels formed?Hydrogels are formed by either physical or chemical crosslinking of polymers. Physical crosslinking involves forming links via electrostatic attraction, hydrogen bonds, or hydrophobic interactions. Chemical crosslinking involves forming covalent bonds through polymerization and copolymerization methods.
Are hydrogels biodegradable?Some hydrogels can be biodegradable, especially those made from natural materials. The biodegradability of hydrogels depends on their chemical composition and can be designed to degrade in the body over time, making them suitable for medical applications like drug delivery.
Are hydrogels safe?Generally, hydrogels are safe and non-toxic. However, their safety can depend on their specific composition and how they are used. For example, in medical applications, they are designed to be biocompatible and not cause adverse reactions in the body.
Can hydrogels be used for drug delivery?Yes, hydrogels are widely used in controlled drug delivery systems. Due to their high water content and biocompatibility, they can carry drugs and release them in the body in a controlled manner over time.
What is the future of hydrogels?The future of hydrogels is promising, with many potential applications in fields like regenerative medicine, tissue engineering, and robotics. Researchers are exploring ways to make hydrogels more durable, responsive to stimuli, and suitable for various uses.
How are hydrogels different from other gels?Hydrogels are distinct from other gels primarily due to their high water content and their unique properties derived from it. Unlike oil-based or alcohol-based gels, hydrogels can absorb and retain large amounts of water, which gives them their soft and pliable characteristics.
|
|
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
