| Mar 06, 2024 | |
Silicon nanotube bundles facilitate efficient RNA therapy delivery in cells |
|
| (Nanowerk Spotlight) Precisely modulating gene expression within cells represents a powerful frontier in treating diseases at their roots, rather than merely managing symptoms. Small interfering RNA (siRNA) molecules have emerged as a versatile tool capable of silencing specific genes by disrupting production of their protein products. However, safely and effectively delivering these delicate RNA strands into cells has remained an obstinate challenge, hindering the broad clinical translation of RNA therapeutics. | |
| Traditional delivery methods relying on viral vectors or synthetic polymers have frequently faced roadblocks like toxicity, immune reactions, and inadvertent off-target effects. This impasse has galvanized researchers to explore innovative nanotechnology-based platforms that could ferry siRNA payloads into cells more precisely and with minimal collateral damage. | |
| This hurdle has spurred extensive research into alternative delivery platforms, particularly those leveraging nanotechnology. Nanostructured materials like porous silicon have garnered significant attention due to their biocompatibility, tunable properties, and potential for surface modifications to facilitate targeted delivery. While early efforts focused on microparticles and nanoparticles, recent advancements have enabled the exploration of more complex morphologies, such as nanowires and nanotubes, which could offer distinct advantages. | |
| In a recent study published in Nano Select ("Porous silicon nanotube bundles as nanocarriers for small interfering RNA delivery"), researchers from Texas Christian University have demonstrated the potential of silicon nanotube bundles as efficient nanocarriers for siRNA delivery into human cervical cancer (HeLa) cells. Their work builds upon the team's previous development of straightforward methods for producing these one-dimensional nanostructures through sacrificial templating techniques. | |
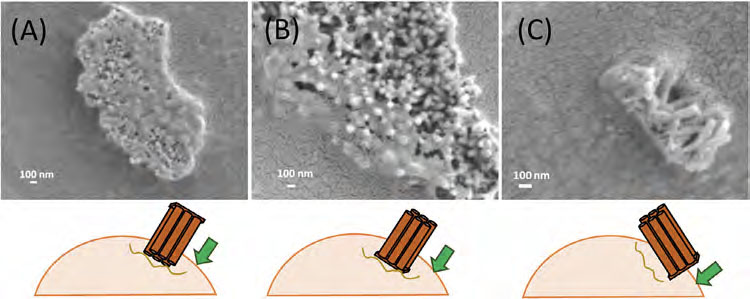 |
|
| SEM imaging showing siRNA/APTES-pSiNT bundles positioned on the HeLa cells via: (A) closed-ends; (B) open ends, and (C) sideways orientation after 4-h incubation. (© Nano Select) | |
| The researchers synthesized porous silicon nanotubes (pSiNTs) by depositing silicon onto zinc oxide nanowire templates and subsequently removing the templates. These pSiNTs were then surface-modified with aminopropyltriethoxysilane (APTES), which enabled the electrostatic conjugation of siRNA molecules targeting the expression of Enhanced Green Fluorescent Protein (eGFP) in the HeLa cells. | |
| Through a combination of imaging techniques, including scanning electron microscopy (SEM) and confocal fluorescence microscopy, the researchers observed the successful internalization of the siRNA-loaded nanotube bundles into the cells. Remarkably, their approach achieved around 50% knockdown of eGFP expression compared to control cells, demonstrating the efficacy of the pSiNT platform in delivering functional siRNA payloads. | |
| While the knockdown efficiency was lower than that achieved by a commercial transfection reagent used as a benchmark, the researchers highlighted several advantages of their nanotube-based system. First, the pSiNTs exhibited negligible cytotoxicity, suggesting good biocompatibility. Additionally, the researchers observed signs of degradation of the nanotube bundles within the cells over time, indicating their potential for biodegradability and clearance from the body – a crucial consideration for clinical applications. | |
| The study also provided insights into the interactions between the nanotube bundles and the cells. SEM imaging revealed that the cell membranes readily wrapped around the bundles, facilitating their internalization. Interestingly, the researchers observed that shorter nanotubes (submicron lengths) appeared to be more favorable for efficient cellular uptake. | |
| While these initial findings are promising, the researchers acknowledged several challenges and opportunities for future investigations. Improving the conjugation efficiency and siRNA loading capacity of the pSiNTs could further enhance their delivery performance. Additionally, gaining a deeper understanding of the cellular uptake mechanisms and exploring strategies to target specific cell types or tissues could expand the platform's therapeutic potential. | |
| Overall, this study highlights the exciting potential of sacrificial template-derived silicon nanotubes as nanocarriers for RNA therapeutics. By leveraging the unique properties of these one-dimensional nanostructures, researchers are expanding the library of possible nanostructures for targeted drug delivery, paving the way for more effective and safer treatments for a wide range of diseases. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
