| Apr 05, 2024 | |
Scientists develop novel method to control the geometry of chiral gold nanoparticles using copper ions |
|
| (Nanowerk Spotlight) Noble metal nanoparticles like gold and silver have been extensively studied for their optical, electronic, and catalytic properties, which can be finely tuned by controlling their size, shape, and composition. However, creating nanoparticles with specific geometries and desired properties has remained a significant challenge, limiting their practical applications. | |
| One area of particular interest is the development of chiral nanoparticles. These are nanoparticles that possess a unique form of asymmetry known as chirality. This means they have a specific 'handedness,' similar to how your left and right hands are mirror images but cannot be perfectly overlaid onto each other. In the context of nanoparticles, this refers to their ability to exist in two forms that are mirror images of each other but cannot be superimposed. | |
| Chiral nanoparticles have shown promise in applications such as sensing, catalysis, and photonics due to their ability to interact differently with left and right circularly polarized light. However, the precise control over the chirality of nanoparticles has been limited by the lack of understanding of the underlying mechanisms that govern the transfer of chirality from molecules to nanoparticles during synthesis. | |
| In recent years, seed-mediated growth has emerged as a powerful approach for creating noble metal nanoparticles with controlled sizes, shapes, and compositions. This method involves the use of small nanoparticle seeds that serve as nucleation sites for the growth of larger nanoparticles. By carefully controlling the growth conditions, such as the concentration of precursors, surfactants, and additives, researchers have been able to create a wide variety of nanoparticle shapes, including rods, cubes, and octahedra. However, the use of foreign additives, such as metal ions, to control the chirality of nanoparticles during seed-mediated growth has remained largely unexplored. | |
| Now, in a recent study published in the Journal of the American Chemical Society ("Cu2+-Dominated Chirality Transfer from Chiral Molecules to Concave Chiral Au Nanoparticles"), a team of researchers from Wuhan University in China has developed a new approach for creating chiral gold nanoparticles with concave surfaces and enhanced optical and catalytic properties. Led by Prof. Qingfeng Zhang, the team demonstrated that the introduction of copper ions (Cu2+) during the seed-mediated growth of gold nanoparticles can dramatically alter their geometric evolution and lead to the formation of concave chiral nanoparticles with enriched geometric chirality. | |
 |
|
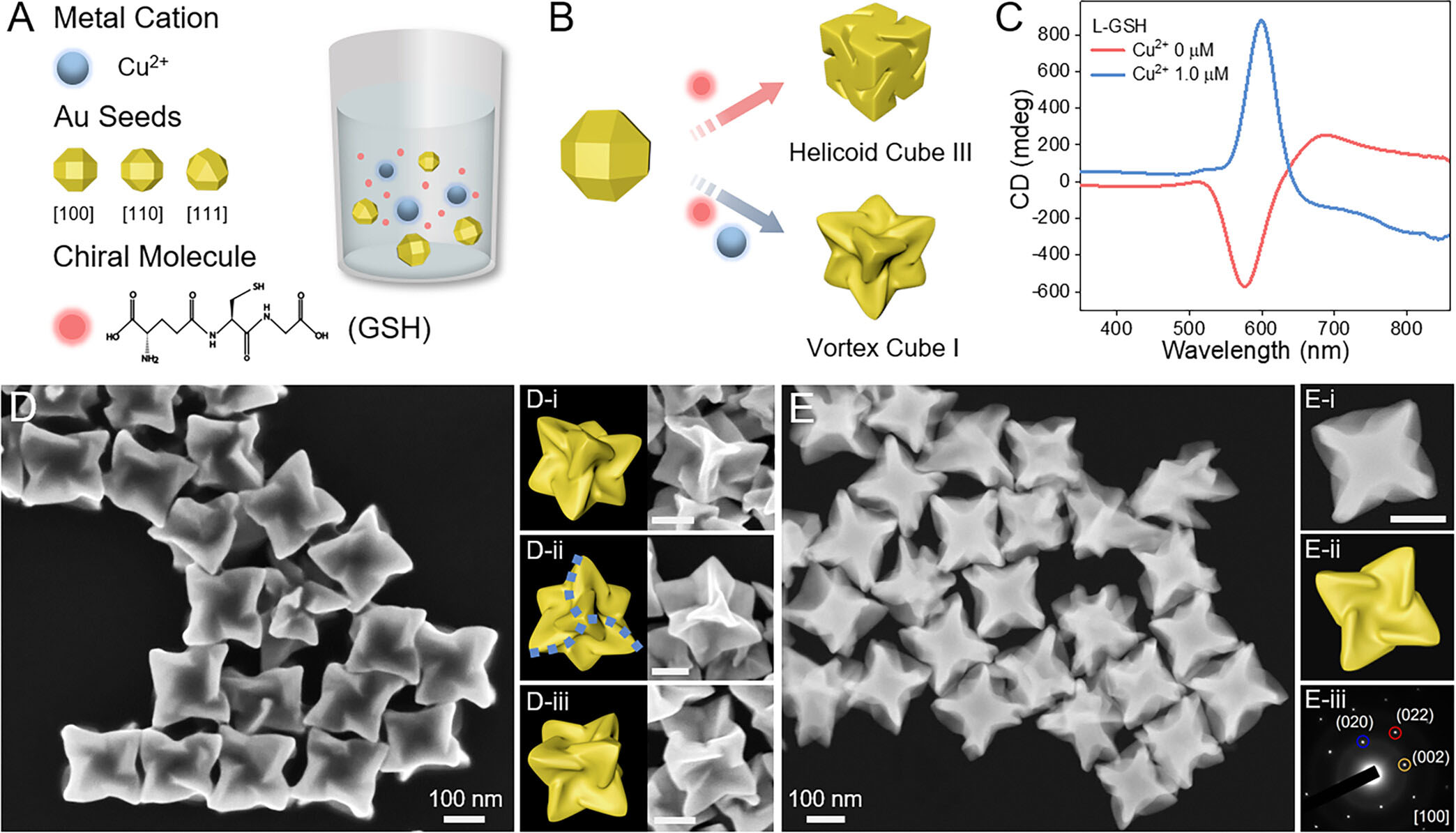
| Cu2+-dominated seed-mediated chiral growth of concave chiral Au VC-I NPs. VC-I: Vortex cube I. (A) Schematic illustration of the Cu2+-dominated seed-mediated chiral growth protocol. Au seeds: Single-crystalline Au RCO NPs. GSH: Glutathione. (B) Schematic illustration of the geometric transformation of chiral Au NPs synthesized in the presence of GSH with and without adding Cu2+. HC-III: Helicoid cube III. (C) CD spectra of chiral Au HC-III (red curve, without Cu2+) and VC-I (blue curve, with Cu2+). (D) SEM image of chiral Au VC-I. The structural features of chiral Au VC-I were further characterized by the SEM images and the corresponding geometric models of an individual D-(i−iii) chiral VC-I. (E) HAADF-STEM image of chiral Au VC-I. HAADF-STEM image (E-i), geometric model (E-ii), and the corresponding SAED pattern (E-iii) of a single chiral VC-I. Scale bars in panels (D, E): 100 nm. (Reprinted with permission by American Chemical Society) | |
| The researchers began by synthesizing single-crystalline gold rhombicuboctahedron nanoparticles to serve as seeds for the chiral growth process. They then introduced the seeds into a growth solution containing gold precursors, surfactants, reducing agents, and chiral molecules, such as glutathione or cysteine. | |
| "Surprisingly, we found that the addition of trace amounts of Cu2+ ions to the growth solution dramatically alters the outcome of the chiral growth process, leading to the formation of concave chiral nanoparticles with a distinct vortex cube geometry," Zhang tells Nanowerk. | |
| Through a series of careful experiments and theoretical modeling, the researchers demonstrated that the Cu2+ ions play a dominant role in guiding the chiral growth of the nanoparticles by selectively activating the deposition of gold atoms along specific crystallographic directions. This selective deposition leads to the formation of concave surfaces with high-index facets, which are known to be more catalytically active than the corresponding low-index facets. | |
| High-index facets refer to the specific crystallographic planes of a nanoparticle that have a high Miller index. The Miller index is a notation system in crystallography that describes the orientation of planes in a crystal lattice. High-index facets are surfaces with complex, often more 'stepped' and 'kinked' geometries compared to low-index facets, which are flatter and smoother. These high-index facets are known to be more catalytically active due to their increased surface area and the presence of more reactive sites, making them highly desirable for applications in catalysis. | |
| The researchers further explored the optical properties of the concave chiral nanoparticles using a combination of experimental measurements and numerical simulations. They found that the concave chiral nanoparticles exhibit significantly enhanced circular dichroism signals compared to their convex counterparts, indicating a stronger interaction with circularly polarized light. | |
| Circular dichroism is a form of light absorption that occurs differently depending on the polarization of the light. Specifically, it refers to the phenomenon where chiral molecules – or in this case, nanoparticles – absorb left- and right-handed circularly polarized light to different extents. This property is crucial for applications in sensing and photonics, where the interaction with light of different polarizations can be used for detection or manipulation of materials. | |
| The origin of this enhanced chirality was attributed to the unique geometric arrangement of the concave surfaces, which creates a distinct chiral pattern of surface plasmon resonances. | |
 |
|
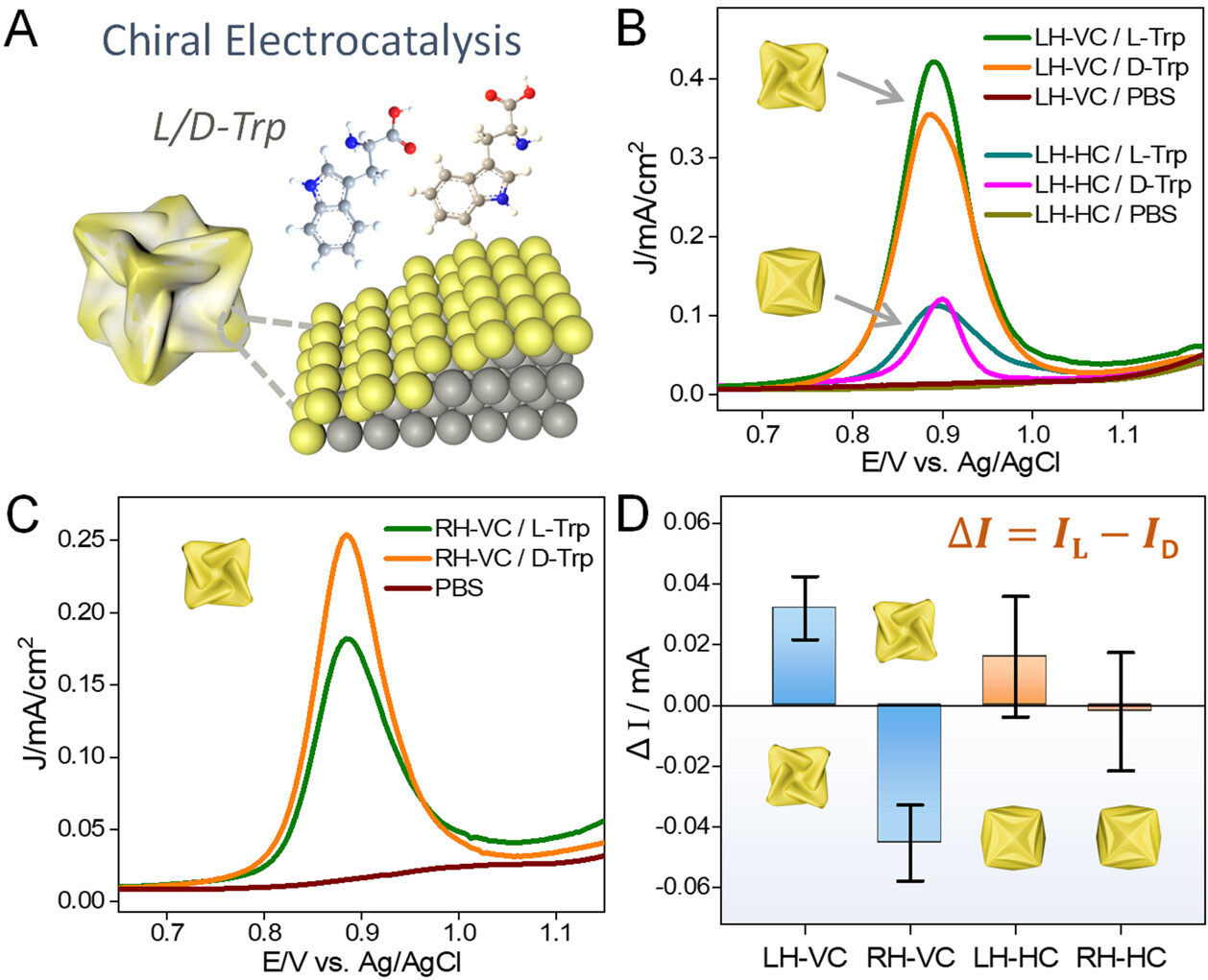
| Chiral electrocatalytic activities of concave chiral VC Au NPs toward the electrooxidation of the tryptophan (Trp) enantiomer. (A) Schematic illustration of the electrooxidation of l/d-Trp using concave chiral VC-III NPs. (B, C) Differential pulse voltammogram (DPV) curves of 1 mM l/d-Trp using (B) LH-VC-III and LH-HC-I and (C) RH-VC-III electrodes in 0.1 M PBS solution (pH = 7.4), respectively. (D) Comparison of the chiral catalytic activities toward the electrooxidation of Trp using LH/RH-VC-III and LH/RH-HC-I NPs. LH/RH: left/right-handed. (Reprinted with permission by American Chemical Society) | |
| To demonstrate the practical utility of the concave chiral nanoparticles, the researchers investigated their catalytic activity towards the electrooxidation of amino acids, which are important chiral molecules in biology and medicine. They found that the concave chiral nanoparticles exhibit significantly enhanced catalytic activity and selectivity towards the oxidation of specific amino acid enantiomers compared to their convex counterparts. This enhanced activity was attributed to the high density of atomic steps and kinks on the concave surfaces, which provide more active sites for catalysis. | |
| The development of this Cu2+-dominated chiral growth strategy represents a significant advance in the field of nanomaterials synthesis and opens up new avenues for creating chiral nanoparticles with tailored properties for a wide range of applications. By providing a simple and versatile approach for controlling the chirality and surface structure of nanoparticles, this work could lead to the development of new catalysts, sensors, and photonic devices with enhanced performance and specificity. | |
| Moreover, the insights gained from this study could also shed light on the fundamental mechanisms underlying the transfer of chirality from molecules to nanoparticles, which has remained a long-standing mystery in the field. By elucidating the role of foreign ions in guiding the chiral growth process, this work provides a new framework for understanding the complex interplay between surface chemistry, crystallography, and geometric structure in determining the properties of chiral nanoparticles. | |
| "Looking forward, we envision that our Cu2+-dominated chiral growth strategy could be extended to other noble metals and materials, opening up new possibilities for creating chiral nanostructures with novel properties and functions," Zhang concludes. | |
| As the field of nanomaterials continues to evolve and mature, the ability to precisely control the chirality and surface structure of nanoparticles could enable a new generation of advanced materials with unprecedented performance and functionality. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
