| Mar 19, 2024 | |
Gold nanocatalysts cross blood-brain barrier aided by unique protein corona composition |
|
| (Nanowerk Spotlight) The blood-brain barrier, a tightly regulated interface between the circulating blood and the central nervous system, has long been a daunting obstacle in the quest to deliver effective treatments for neurodegenerative diseases. Despite significant advancements in our understanding of these debilitating conditions, the development of therapies capable of penetrating this protective barrier and reaching the affected areas of the brain has remained an elusive goal. | |
| In the face of this challenge, researchers have explored a wide range of strategies, from small molecule drugs to antibodies and nanoparticles, in an attempt to breach the blood-brain barrier. While some of these approaches have shown promise, they have often been hampered by limitations such as low efficacy, off-target effects, and safety concerns. As the global burden of neurodegenerative diseases continues to grow, the need for innovative solutions has become increasingly urgent. | |
| Enter nanotechnology, a field that has revolutionized drug delivery and opened up new possibilities for targeting the brain. Among the various types of nanoparticles being investigated, gold nanoparticles have emerged as a promising contender due to their biocompatibility, ease of synthesis, and versatility in surface functionalization. These unique properties have made them an attractive candidate for delivering therapeutic agents across the blood-brain barrier. | |
| A groundbreaking study published in ACS Pharmacology & Translational Science ("Protein Corona Composition of Gold Nanocatalysts") has shed new light on the mechanisms by which a specific type of gold nanocrystals, known as CNM-Au8®, a registered trademark of Clene Nanomedicine, Inc., can effectively traverse the blood-brain barrier and target human brain tissue for treating neurodegenerative disorders. Developed by Clene Nanomedicine, Inc., CNM-Au8 represents the first gold nanocrystal suspension being actively explored as a therapeutic drug for conditions such as multiple sclerosis, amyotrophic lateral sclerosis, and Parkinson's disease. | |
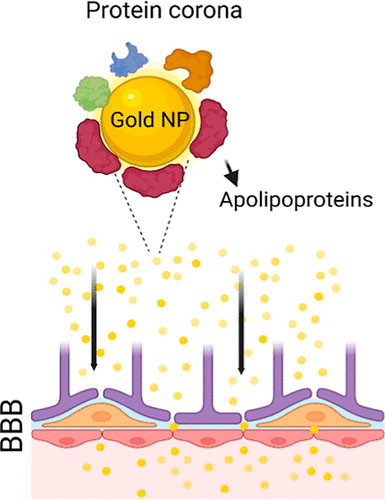 |
|
| Graphical abstract of the work. (Image: ACS Pharmacology & Translational Science, CC-SA 4.0) | |
| The researchers, led by Morteza Mahmoudi, conducted a detailed investigation of the protein corona profiles of CNM-Au8 gold nanocrystals to gain insights into their prolonged blood circulation time and ability to cross the blood-brain barrier. Unlike traditional methods of producing colloidal gold nanoparticles, which rely on surface-capping organic ligands for crystal growth and stability, CNM-Au8 is synthesized without these ligands. This unique property not only ensures clinical safety and tolerability in human subjects but also enhances its biocompatibility profile. | |
| Upon interaction with human plasma, the researchers discovered that CNM-Au8 gold nanocrystals effectively attract a variety of crucial apolipoproteins, notably apolipoproteins E, to their surfaces. This interaction likely facilitates their passage through the blood-brain barrier. Apolipoproteins play a crucial role in this process through two main mechanisms. | |
| "Firstly," as Mahmoudi explains to Nanowerk, "certain apolipoproteins, such as apolipoprotein E, can bind to specific receptors on the blood-brain barrier, promoting receptor-mediated transcytosis. Secondly, apolipoproteins can mimic the low-density and high-density lipoproteins that are naturally transported across the barrier, thereby enhancing the uptake and transport of CNM-Au8 nanocrystals into the brain." | |
| The study revealed that over 80% of the apolipoproteins participating in the protein corona composition of CNM-Au8 are comprised of five subtypes: apolipoproteins A-I, A-II, C-II, C-III, and E. These apolipoproteins work synergistically to enhance the nanoparticles' ability to cross the blood-brain barrier. For instance, apolipoprotein A-I can traverse the barrier via clathrin-independent and cholesterol-mediated endocytosis, while the presence of apolipoprotein A-I specifically bound to apolipoprotein C-III demonstrates a notable correlation with cerebrospinal fluid apolipoprotein C-III levels. | |
| Furthermore, the protein coronas of CNM-Au8 nanocrystals exhibit a substantial presence of albumin and a notable absence of opsonin-based proteins. This composition contributes to prolonged blood circulation by reducing the potential for nanoparticle eradication by the immune system. The lack of accumulated absorption of high amounts of immunoglobulin and fibrinogen chains also suggests the absence of a fibrillation process that some proteins undergo after interaction with gold nanoparticles. | |
| The implications of these findings are significant for the development of advanced therapeutic agents aimed at combating neurodegenerative diseases. The selective binding of apolipoproteins to CNM-Au8 nanocrystals, particularly apolipoproteins A-I, A-II, C-II, C-III, and E, demonstrates their pivotal role in facilitating nanoparticle transport through the blood-brain barrier. These critical factors, combined with the absence of significant levels of complement proteins and fibrinogens in the protein corona, contribute to the therapeutic potential of CNM-Au8 in targeting neurodegenerative diseases. | |
| The innovative and patented electrochemical synthesis approach of CNM-Au8, free from surface-capping organic ligands, not only ensures safety and tolerability but also plays a key role in shaping its unique protein corona composition. This aspect is particularly relevant in the context of clinical applications, as evidenced by ongoing clinical trials. The distinctive protein corona of CNM-Au8 gold nanocatalysts opens new avenues for the development of nanoparticle-based therapies, particularly for neurodegenerative conditions, where blood-brain barrier penetration is a significant challenge. | |
| As research continues to unravel the complexities of the blood-brain barrier and the potential of nanoparticle-based drug delivery systems, the findings of this study provide valuable insights into the mechanisms underlying the therapeutic efficacy of CNM-Au8 gold nanocatalysts. The selective enrichment of apolipoproteins in their protein corona, coupled with the absence of significant immune response triggers, highlights the importance of tailoring nanoparticle properties to achieve desired biological interactions and therapeutic outcomes. | |
| "While further research is needed to fully understand the long-term safety and efficacy of CNM-Au8 and other nanoparticle-based therapies for neurodegenerative diseases, the results of our study represent a significant step forward in the quest to overcome the blood-brain barrier and deliver effective treatments to the brain," Mahmoudi concludes. | |
| As the scientific community continues to explore the potential of nanotechnology in medicine, the unique properties and protein corona composition of CNM-Au8 gold nanocatalysts serve as a promising example of the innovative strategies being developed to address the challenges posed by neurodegenerative disorders. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
