| Nov 19, 2020 | |
Morphing 2D circuits - next-generation microfluidics gets rid of solid walls |
|
| (Nanowerk Spotlight) Many microfluidic devices used in life sciences employ plastics or silicones as a base material. These devices are usually prototyped by engineers and go through a lengthy and complex manufacturing process before being used by researchers. More importantly, biological samples are physically and optically inaccessible, due to the surrounding solid walls which limits their usefulness downstream; for example retrieving specific cell for downstream assays can be challenging. | |
| "This is part of a larger issue with microfluidic devices in life sciences: most platforms rely on materials or chemicals that are outside the biological breadth as building blocks, scaffolds, or coatings for the devices," Ed Walsh, an Associate Professor in Engineering Science at Oxford University's Thermofluids Institute, tells Nanowerk. "As the title of a reference article in the field states, "Engineers are from PDMS-land, Biologists are from Polystyrenia", meaning that there is a major disparity between what biologists need and what engineers design." | |
| In a game-changing approach, Walsh and his collaborators from engineering and biomedical fields are developing a transformative way to fabricate microfluidic devices, where solid walls are replaced by transparent, morphing fluid walls using only biocompatible materials. | |
| A new paper in Advanced Science ("Jet-Printing Microfluidic Devices on Demand") describes the team's novel method to generate cell-friendly microfluidic devices on demand with features <50 µm in size. | |
| This innovative protocol offers significant benefits to scientists in biology and biomedicine who can now easily create their own cellular microfluidic environments in minutes – almost as quickly as the circuit pattern can be drawn on paper – using truly cell-friendly materials, standard Petri dishes and culture media. | |
| "The core idea behind our technology is that in the micro-world interfacial forces dominate gravitational ones, so fluid interfaces can act as robust walls for microscale structures," Walsh explains. "We use an immiscible, bio-inert fluorocarbon, FC40, to shape two immiscible fluids into micro-environments of interest to biologists. The newly formed micro-environments reside on conventional cell-culture dishes and are fully accessible for optical and physical investigation due to their transparent, morphing walls." | |
| "Moreover" he adds, "they do not suffer from the catastrophic gas-bubble issues so commonly found in traditional microfluidics; with fluid walls gas bubbles are automatically released to the atmosphere via naturally occurring buoyancy forces." | |
 |
|
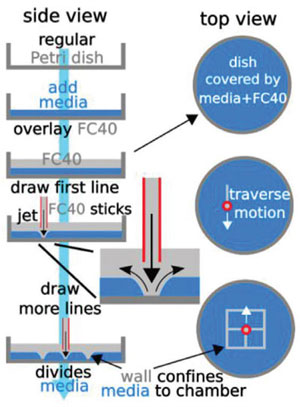
| Principle of the technique: A 'microjet' of an immiscible fluorocarbon (FC40) is projected through FC40 and media on to the bottom of a dish; media is pushed aside, and FC40 sticks to the bottom (it wets polystyrene better than media). Moving the jet now draws lines to form a grid with FC40 walls. (Reprinted with permission from Wiley-VCH Verlag) | |
| Why do we build objects from solids rather than fluids? The authors of this Science Advances paper have pondered this question for years. The answer: At the length scales that humans interact with objects gravity dominates; and anything one might make from fluids at this scale would collapse into a puddle on the ground. | |
| "When things get much smaller the physics is different, gravitational forces becomes irrelevant and interfacial forces dominant," Walsh points out. "We exploit this physics to create objects from fluids, from fluid pumps to microfluidic flow networks, and thereby deliver a new technology platform that is shaping future discoveries in biology through shaping immiscible fluids." | |
| Imagine writing on paper, except instead of a pen delivering ink you have a micro-needle infusing a water-immiscible fluid (fluorocarbon), into a petri dish with a thin film of cell media already present. The fluorocarbon (fluid) walls stay pinned to the polystyrene surface, the same way ink sticks to paper, enclosing aqueous structures. | |
| This way the team can create a series of microfluidic chambers, or any micro-environment, that are isolated from each other by liquid walls of FC40. Chambers may then used like wells in microplates: liquids are added/removed to/from them by pipetting through FC40 instead of air. Or fluid walled conduits can be used to pump fluids through them just like their pipe networks solid walled counterparts. | |
| The researchers also introduced a 3-axis traverse to carry the infusing needle, which makes fabrication faster and more precise the same way a printer automates handwriting. | |
| Fluids find path through a maze with a single inlet and outlet. The circuit was built by jetting FC40 through media plus red dye; when blue dye is pumped into the inlet, it takes the path of least resistance through the maze. (Video: Oxford Thermofluids Institute) | |
| This new technology empowers biologists to shape micro-environments for their experiments easily. | |
| For instance, in cell biology samples, cell media and reagents are stored in conventional microtiter plates. Although they come in several standard formats, they work with volumes a few orders of magnitude higher than what samples actually need. | |
| In contrast, the team's new on-demand jet-printing technology allows biologists to quickly build their own multi-well plates at a more physiologically-relevant scale for their samples. A Petri dish can be repurposed into a 256- or 1024-well plate in less than 5 minutes. | |
| This on-demand fabrication can be extended to a wide range of dynamic microfluidic circuits with flow, previously possible only using specialized manufacturing processes, with inherent operational limitation, that can take weeks. For instance, this technology is able to accelerate single cell cloning workflows in cell biology significantly: from 1-2 months, down to 7-8 days. This is currently automated using the isoCell, a benchtop device commercialized by iotaSciences, a company which also supports this research. | |
| The authors demonstrate that the strength of the fluorocarbon jet can be adjusted to serve multiple functions for biological assays. For example, the FC40 jet strength can be adjusted to dislodge adherent cells from a dish (think of a jet-hose cleaning the bottom of a pool), while not sufficiently strong to form stable fluid walls. This is demonstrated in the video below, or at another setting it may be used to mix the tiny volumes in the micro-environments (see paper). | |
| Creating a fluidic wormhole. Using a microjet to create a vortex in fluids to dislodge, extract, and replate cells in microfluidic devices. (Video: Oxford Thermofluids Institute) | |
| The team is already expanding the application space of their platform technology with biological research groups within the University of Oxford. Some application areas they are focussed on include chemotaxis (the response of cells to chemical gradients); antibacterial resistance; cell-cell interaction; 3D cultures; and development of 'organ-on-a-chip' models to better recapitulate human biology in a dish. | |
| In addition, the technology is currently finding routine use outside of Oxford University in academic and industry labs with a diverse range of applications involving CRISPR, gene editing and a vast range of cell lines from cancer cells to human induced Pluripotent Stem Cells (hiPSCs). | |
| "At a theoretical level, unlike flow through a pipe, where there is a solid theoretical framework in place developed over decades, to guide experiments, flow through channels with fluid walls represents an unchartered territory in engineering," Walsh concludes. "We are dealing with a system where the walls are morphing continuously to accommodate changes in pressure, which makes this a complex, but exciting, problem to look at." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
