| Apr 12, 2021 |
Chemists create renewable plant-based polymers
|
|
(Nanowerk News) Researchers at the Laboratory of Cluster Catalysis at St Petersburg University have synthesised polymers from biomass. What makes them different is that they can be easily recycled (Green Chemistry, "Biomass- and calcium carbide-based recyclable polymers").
|
|
Today, our life is simply unthinkable without polymers. Plastics, fibres, films, paint and lacquer coating - they are all polymers. We use them both in our everyday life and in industry. Yet the goods made from polymers, e.g. bottles, bags, or disposable tableware, are used just once or for a short period of time before they are thrown away. Due to the chemical compounds that they may release during recycling, they pose a real threat to our environment.
|
|
There are few polymers that can be recycled many times. This stirs up interest in secondary recycling. However, the goods made from secondary raw materials are lower in quality compared to the goods from primary raw materials.
|
|
The new polymers are based on biomass compounds. Biomass is a renewable source of raw materials for the chemical industry of the future. The key component of these polymers is terpenols, i.e. compounds from natural alcohols. Among them are such well-known examples as: menthol derived from the essential oil in mint; and borneol - a large quantity of which can be found in the essential oil in the white fir tree.
|
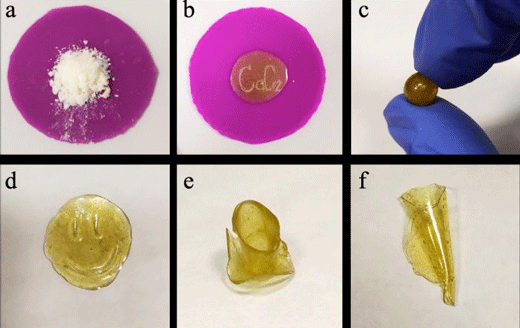 |
| Mechanical treatment of mitranol-based polymers: a) primary polymer as white powder; b) melted polymer, c-f) various forms of the polymer after being melted repeatedly. (Image: SPbU)
|
|
The synthesised polymers may well be used for primary and secondary recycling. During secondary recycling, the polymer-based products can be converted into the primary compounds. This may be further followed by polymerisation. These polymers can be recycled at moderate temperatures.
|
|
'This can be said about recycling the materials based on our polymers. If they are recycled without oxygen, we can get natural alcohols or their derivatives that can be restored to the same alcohols. Because they are widely found in nature, they do not harm the environment,' said Svetlana Metlyaeva, the first author of the article and a researcher at the Laboratory of Cluster Catalysis at St Petersburg University.
|
|
The polymers of this type can be melted at about 120 °C and shaped in another way, she said. When cooling, they become hard. Interestingly, the chemists repeated this cycle seven times and concluded that the polymers, when melted more than once, did not change their properties.
|
|
The researchers are planning to continue their work at the Research Park at St Petersburg University. They will study the mechanical properties of the polymers, including resilience, elasticity, strength, and others. This is an important step towards our understating of how to use them in industry.
|
|
'What we have achieved so far is only the ability to synthesise these polymers. Yet the properties of the polymer-based materials can vary. This depends on the way in which we synthesise them and what compounds we use. Now we have to modify the polymers themselves and the materials based on them. Then we will be able to talk about how we can use them,' said Svetlana Metlyaeva.
|

