| Posted: May 26, 2010 | |
New findings about the activity of graphene oxide sheets at interfaces |
|
| (Nanowerk Spotlight) Graphite oxide sheet, now being named graphene oxide – a compound of carbon, oxygen, and hydrogen obtained by treating graphite with strong oxidizers – has attracted substantial interest as a possible intermediate for the manufacture of graphene. It has been largely viewed as hydrophilic – having a strong affinity for water illustrated by its excellent colloidal stability in water. In what should help to better understand and improve the solution processing of graphite oxide-based graphene materials and open up opportunities to design new functional graphite oxide-based hybrid materials, researchers have now reported that graphite oxide is amphiphilic – possessing both hydrophilic and lipophilic (fat-liking) properties – and can adsorb onto air-water, oil-water and solid-water interfaces. | |
| "Graphene oxide has largely been viewed as hydrophilic since its first discovery in the 19th century" Jiaxing Huang, an assistant professor at Northwestern's Department of Materials Science & Engineering, tells Nanowerk. "We had some doubts about this popular view. Graphene oxide has functional groups on its edges that can be negatively charged, which makes it disperse well in water. However, it still possesses graphene nanodomains on its basal plane, which should be hydrophobic." | |
| This motivated the researchers to view graphene oxide as an amphiphile, or surfactant, which should show interfacial activities. | |
| Reporting their findings in the May 19, 2010 online edition of Journal of American Chemical Society ("Graphene Oxide Sheets at Interfaces"), Huang and his co-workers found that graphene oxide can indeed act like a surfactant, as measured by its ability to adsorb on air-water, oil-water and solid-water interfaces, and lower the interfacial tension. | |
 |
|
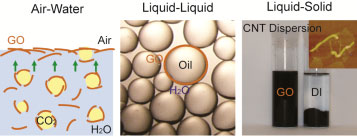
| Graphene oxide sheets can (left) accumulate at water surface by flotation, (middle) stabilize organic solvent in water and (right) disperse carbon nanotubes in water, thus acting as surfactant. (Image: Jiaxing Huang, Northwestern University) | |
| First, the team found that graphene oxide can be lifted onto a water surface by flotation. This was demonstrated by putting graphene oxide in carbonated water. After adding some boiling stones, graphene oxide is transported to the surface by the carbon dioxide bubbles. | |
| A particular interesting discovery in the Northwestern paper is that graphene oxide's amphiphilicity is size dependant – smaller graphene oxide pieces are more hydrophilic. When a mixture of large and small pieces is spread on a water surface, small ones sink while larger ones float. This means a water surface can be used as a filter for size separation. | |
| Next, the team found that graphene oxide can stabilize oil droplets in aqueous phase, forming Pickering emulsions that are stable for months. | |
| Finally, the team demonstrated the use of graphene oxide as surfactant to disperse graphite and carbon nanotubes in water. | |
| "A tremendous amount of effort has been devoted to making carbon nanotubes (CNTs) water processable through wrapping by water-soluble materials," says Huang. "Since many surfactants for dispersing CNTs have polyaromatic components we hypothesized that graphene oxide should be able to adhere to CNTs and disperse them in water as well." | |
| And indeed, CNTs become disentagled and disperse quite well in graphene oxide water after sonication. | |
| Since it can be readily reduced to conductive, chemically modified graphene, graphene oxide could be a particularly attractive dispersing agent for solution processing of materials for electronic applications. | |
| "Now the surfactant itself is a functional component as well" says Huang. "Commonly used dispersing agents such as molecular surfactants, polymers, and DNA are usually insulating materials, which need to be removed afterward to avoid decreased conductivity. However, graphene oxide can actually provide more conducting pathways in the final complex after it is reduced." | |
| Huang concludes that the new knowledge of the amphiphilicity of graphene oxide should lead to a better understanding of and improvements in its solution processing. It could lead to the discovery of many more interesting self-assembly behaviors of these 2D sheets, particularly at interfaces. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
