| Posted: Oct 31, 2011 | |
Argument that catalyst poisoning halts carbon nanotube growth needs reexamination |
|
| (Nanowerk Spotlight) Although a wide variety of methods have been developed to fabricate carbon nanotubes (CNTs), including arc discharge, laser ablation, and chemical vapor deposition (CVD), CVD is the most technically important – since it can be achieved at low temperature and is upscalable – and the most widely used in industry (see Table 2 in our recent Spotlight "Global carbon nanotubes market – industry beckons"). | |
| Using the CVD process, manufacturers can combine a metal catalyst such as iron with reaction gases such as hydrocarbon to form carbon nanotubes inside a high-temperature furnace. This process creates CNTs that are subsequently deposited in a collection environment and harvested into the desired end-product structural form (more on CNT technology: "Delivering on the promise – scaling carbon nanotube technology"). | |
| Understanding the processes in CVD growth of carbon nanotubes is important for their high yield and extended production. But even today, some 20 years since their discovery, the finer details of CNT growth mechanisms remain poorly understood. | |
| One of the issues that manufacturers have to grapple with is the levelling off and ultimate stoppage of CNT growth several minutes into the CVD process. This is referred to as catalyst poisoning – an inactivation or poisoning process of catalyst particles that has been attributed to an overcoat of amorphous carbon. | |
| "Although termination of CNT growth is often attributed to amorphous carbon poisoning, how this actually happens has yet to be demonstrated" Mark Rümmeli, who leads the Molecular Nanostructures group at the Leibniz Institute for Solid State and Materials Research in Dresden, tells Nanowerk. "Previous studies show that controlled amounts of water vapor, or hydrogen/oxygen radicals improve carbon nanotube CVD growth. The argument has been that these species remove or reduce amorphous carbon. However, the argument that water and radicals reduce/remove amorphous carbon has never been demonstrated. In addition, amorphous carbons on contact with catalyst metals are implemented for graphene formation. This later point contradicts the theory of catalyst poisoning by amorphous carbon when growing carbon nanotubes in CVD." | |
| In a recent paper in the October 24, 2011 online edition of ACS Nano ("Catalyst Poisoning by Amorphous Carbon during Carbon Nanotube Growth: Fact or Fiction?"), Rümmeli and his team, with collaborators from TU Dresden, University of Oxford, and University of Dayton, reveal that the presence of amorphous carbon does not prevent catalytic hydrocarbon decomposition and graphitization processes. There are two key findings in this work: | |
| – Amorphous carbon does not poison metal catalyst particles during thermal CVD growth of carbon nanotubes. The study shows continued carbon nanotube growth even when exposed to large amounts of amorphous carbon and carbon tar. | |
| – Catalyst particles intentionally covered with amorphous carbon prior to a CVD reaction show graphitization; the feedstock is still able to decompose and the catalyst particles are still able to form sp2 carbon. | |
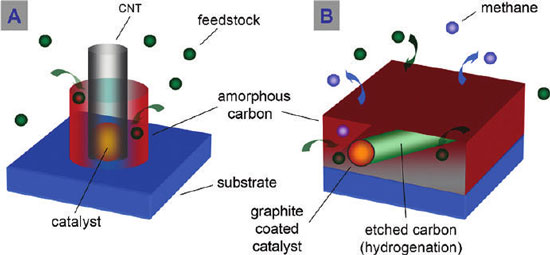 |
|
| (A) Schematic illustrating CNT growth despite the presence of a self-pyrolyzing hydrocarbon feedstock and heavy amorphous carbon deposition. (B) Schematic showing graphitic shell formation around catalyst particles and etching of amorphous carbon by hydrogenation for FePt nanoparticles with a 10 nm deposited amorphous carbon layer and subsequent exposure to a CVD reaction. (Reprinted with permission from American Chemical Society) | |
| "While our studies show that the presence of amorphous carbon does not prevent catalytic hydrocarbon decomposition and graphitization processes, they actually reveal an additional catalytic reaction to be present – catalytic hydrogenation – a process in which carbon in contact with the catalyst surface reacts with hydrogen to form methane," Rümmeli explains. | |
| The researchers note that this process is well-known as a technique to etch crystallographically oriented tracks in graphene. However, in their studies, no tracks are observed; only etched patches are present, which is due to the lack of structural order (amorphous carbon). | |
| "Given our results, the argument that amorphous carbon halts nanotube growth by poisoning the catalyst needs re-examination" says Rümmeli. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
