| Posted: Apr 30, 2012 | |
High-performing supercapacitor electrodes made from self-organizing cobalt oxide nanowires |
|
| (Nanowerk Spotlight) Commercially available supercapacitors store energy in two closely spaced layers with opposing charges and offer fast charge/discharge rates and the ability to sustain millions of cycles. Researchers have come up with various electrode materials – even as exotic as eggshell membranes – to improve the performance of supercapacitors, focussing mostly on porous carbon due to its high surface areas, tunable structures, good conductivities, and low cost. | |
| Based on the charge storage mechanisms, supercapacitors can be divided into two categories – electric double layer capacitors (EDLCs) and pseudocapacitors, which can have higher capacitance values than EDLCs. | |
| "Transition metal oxides (TMOs) are considered as ideal electrode materials for pseudocapacitors as they can provide a variety of oxidation states for efficient redox charge transfer," Husam Alshareef, an associate professor in material science and engineering at King Abdullah University of Science & Technology in Saudi Arabia, explains to Nanowerk. "Although ruthenium oxide (RuO2) has been recognized as the most promising TMO electrode material for pseudocapacitors, its commercial application is restricted due to its high cost, low porosity, and toxic nature." | |
| In a previous Nanowerk Spotlight we reported on earlier supercapacitor electrode research by Alshareef's group ("Carbon nanotube-coated sponge makes an excellent supercapacitor"). | |
| Together with members of his team at KAUST (Dr. R.B. Rakhi and PhD student Wei Chen), Alshareef has now developed novel supercapacitor electrodes with remarkable pseudocapacitance. The team reports its findings in the April 11, 2012 online edition of Nano Letters ("Substrate Dependent Self-Organization of Mesoporous Cobalt Oxide Nanowires with Remarkable Pseudocapacitance"). | |
| Alshareef explains that typically supercapacitor electrodes are made from conducting carbon mixed with a transitional metal oxides that are separately prepared. This mixture is then coated on a highly conducting current collector – carbon cloth, copper or nickel. This approach often limits electrolyte access to the entire electrode volume and limits the surface area where redox reactions can take place. | |
| In contrast, the KAUST team's approach is different because it targets several critical factors simultaneously to enhance device performance. | |
 |
|
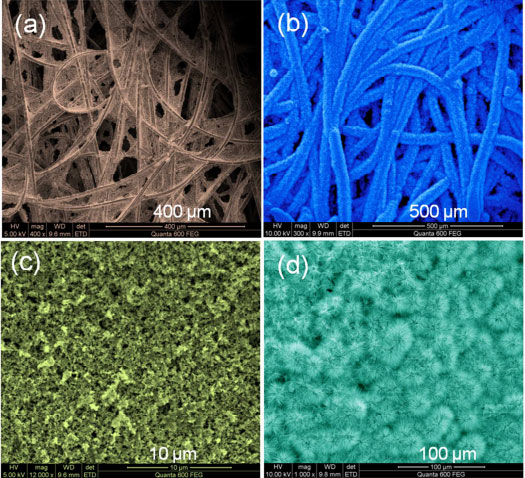
| SEM images of (a) carbon fiber paper (b) Co3O4 nanowires with brush-like morphology, (c) graphitized carbon paper and (d) Co3O4 nanowires with flower-like morphology. (Reprinted with permission from American Chemical Society) | |
| "We designed the electrode with macroporosity to help electrolyte flow and with mesoporosity to increase oxide surface area and increase the redox reaction rate," says Alshareef. "We used a substrate-assisted nucleation process so that every nanowire makes contact with the current collector surface. Our cobalt oxide (Co3O4) nanowires grown on fibrous paper collectors self-organize into a brush-like morphology while the nanowires grown on planar carbon self-organize into a flower-like morphology. The fabricated electrodes offer the desired properties of macroporosity to allow facile electrolyte flow, thereby reducing device resistance while the nanoporosity with large surface area to allow faster reaction kinetics." | |
| Specifically, this novel electrode design combines several ideas: | |
| 1) Using carbon fiber instead of planar carbon to allow more facile electrolyte flow to the entire volume of the electrode; | |
| 2) Using in situ solvothermal synthesis to form mesoporous oxide nanowires directly on the current collector where every single nanowire makes direct contact with the collector surface; | |
| 3) Manipulating the solvothermal process conditions to render the nanowires mesoporous so they have a large surface area. In this study, the researchers use two types of carbon-coated collectors to demonstrate the design concepts using Co3O4 as prototype. | |
 |
|
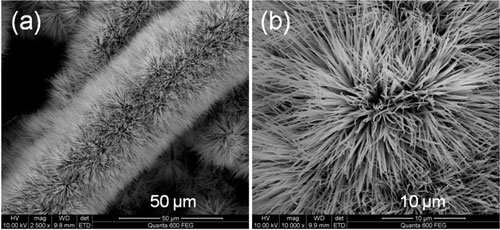
| (a) Low and (b) high magnification SEM images of Co3O4 nanowires with brush-like morphology. (Reprinted with permission from American Chemical Society) | |
| Cobalt oxide (Co3O4) is considered one of the better alternatives to ruthenium oxide due to its high theoretical capacitance and other performance metrics. The Co3O4 nanowires used by the team were grown using solvothermal synthesis directly on either planar carbon-coated or fibrous carbon-coated conductive flexible current collectors. In both cases, the Co3O4 nanowires nucleated starting from the carbon surface, spreading radially out. | |
| Alshareef points out that every single Co3O4 nanowire made contact with the current collector. "The fibrous carbon collector resulted in the oxide nanowire nucleating in a three-dimensional fashion, completely surrounding the fibers, unlike what is the case with planar carbon collectors. In addition, the fibrous carbon structure allowed more facile electrolyte flow. These two effects significantly enhanced the performance of the devices with the fibrous carbon collectors electrodes." | |
| This research appears to solve the main problem of limited capacitor performance by showing a pathway to realizing significant capacitance improvements for oxide-based supercapacitors. The team notes, though, that since cobalt is somewhat expensive they are currently trying the reported approach with other metal oxides that are less expensive in the hope of realizing similar improvements with other oxides. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
