| Posted: Sep 04, 2012 | |
Extremely water repellent graphene foams |
|
| (Nanowerk Spotlight) What happens when a fluid is brought in contact with a solid surface – the process of 'wetting' – has intrigued physicists and material engineers for a long time. In physical terms, the process of wetting is driven by the minimum free energy principle – the liquid tends to wet the solid because this decreases the free energy of the system. Understanding these mechanics, and using nanotechnology to structure surfaces to control wetting, has a far-reaching impact for many objects and applications in our daily lives - anti-sticking pans; low-friction coatings for engine parts; more comfortable contact lenses; better prosthetics; and self-cleaning, anti-fouling or anti-corrosion materials (read more: "Nanotechnology solutions for self-cleaning, dirt and water-repellent coatings"). | |
| The primary measurement to determine wettability is the angle between the solid surface and the surface of a liquid droplet on the solid's surface. For example, a droplet of water on a hydrophobic surface would have a high contact angle, but a liquid spread out on a hydrophilic surface would have a small one. Surfaces where the contact angle is approaching 180° are called superhydrophobic and surfaces where the contact angle is approaching 0° are called superhydrophilic (read more: "'Nailing' superlyophobic surfaces with nanotechnology"). | |
| Researchers have now shown that it is possible to use graphene sheets to create a superhydrophobic coating material that shows stable superhydrophobicity under both static as well as dynamic (droplet impact) conditions. | |
| Reporting their work in the August 22, 2012 online edition of Small ("Superhydrophobic Graphene Foams"), a team led by professors Hui-Ming Cheng from the Shenyang National Laboratory for Materials Science and Nikhil Koratkar at Rensselaer Polytechnic Institute, demonstrates a novel macroscopic graphene structure composed of an integrated foam-like network of graphene sheets with well-controlled microscale porosity and roughness. | |
| "Previous studies, including by my own group, had dispersed graphene in solution and drop cast or spin cast graphene coatings on surfaces to control their wetting," Koratkar tells Nanowerk. "However, we found that under droplet impact conditions such films often fail as the droplet penetrates into the film and gets stuck or pinned into the surface roughness features. We wanted to create a more stable hydrophobic surface with strong entrapped air pockets. So we came up with the idea of the graphene foam which has large air pockets which can be preserved even under conditions of drop impact." | |
| The researchers' novel idea was to grow graphene over a sacrificial nickel foam template and then leech away the nickel, leaving behind a graphene foam with few-layered graphene sheets that comprise the walls of the foam. The foam is then coated with a 200nm layer of Teflon. | |
 |
|
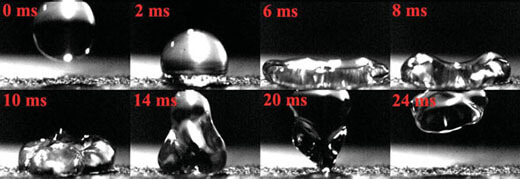
| Snapshots of a water droplet impacting the surface of the Teflon coated graphene foam. The impact velocity just prior to the droplet striking the surface was ∼76 cm/sec. The sequence of snapshots shows the deformation time history of the droplet upon impact. The droplet spreads, then retracts and successfully rebounds off the surface. The coefficient of restitution (i.e. ratio of droplet impacting velocity to ejecting velocity) is ∼0.37 for the Teflon coated foam. (Reprinted with permission from Wiley-VCH Verlag) | |
| Since the graphene foam inherits the pore structure of the nickel foam template, this means that the pore size and structure of the graphene foam can be uniformly tuned by selecting the appropriate nickel foam template. | |
| Koratkar explains that tis type of porous structure excels at trapping air, which gives super-hydrophobicity to the structure. Also, as there are no physical breaks or interfaces in the macroscopic 3D graphene network, the graphene foam is mechanically strong and easy to handle and manipulate. | |
| "This is the first time that graphene has been used in such a systematic way to create super-hydrophobic structures" he adds. | |
| In order to gauge the ability of the foam to repel impacting water drops, the team performed droplet impact tests. | |
| "Droplet rebound is important in lab-on-chip applications where droplet motion is desired and drop pinning is undesirable" explains Koratkar. "Similarly, self-cleaning and anti-fouling surfaces require mobile drops." | |
| In their tests the team found that droplets remains completely intact during the collision and does not splinter into smaller drops. They rebound off the graphene foam without pinning to the surface or damaging the foam structure. | |
| "Interestingly" says Koratkar, "in contrast to the Teflon coated graphene foam, Teflon coated nickel foams did not provide stable superhydrophobic behavior especially under droplet impact conditions. This indicates that the flexibility of the graphene foams could play an important role." | |
| He points out that it would be interesting if one could use electro-wetting to control the wetting in real time: "Since graphene is conductive we can coat it with a dielectric and then use electric field to build charge across the water/graphene interface. This should allow us to control the surface tension of water, which should allow us to control the wettability in real time. This can be useful for creating a gradient in wettability for moving water drops in lab-on-chip applications." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
