| Posted: Jan 11, 2011 | |
Nanotechnology solutions for self-cleaning, dirt and water-repellent coatings |
|
| (Nanowerk Spotlight) A surface is not simply the physical division between an object and its environment; it fulfils a range of functions of its own which often play a crucial part in product design. Surfaces are supposed to feel good to the touch and to look good for as long as possible, be easy to maintain and not be spoiled by dirt, water stains or fingermarks. | |
| Traditional coating materials often do not stand the test of the increased demands made on materials today. In recent years however, advances have been made using methods ascribed to nanotechnology1. | |
| Providing one answer to the question where is nanotechnology used today, the following gives a short overview of the basis of these structures, the processes involved in their manufacture, the areas where they can be used, and the effect they have on the environment and human health. | |
| Self-cleaning I – the "Lotus-Effect" | |
| The lotus plant (Nelumbo nucifera, Figure 1) is revered in Asia for its exceptional cleanness. Although it grows in muddy waters, its leaves always appear immaculately clean. The plants' leaves are superhydrophobic, i.e. drops of water roll off free of residue, taking any impurities with them. | |
 Figure 1: The lotus plant (Nelumbo nucifera). Investigations into the surface using reflection electron microscopy (REM) have shown that the surface of the leaf is not especially even, which you might intuitively assume, but has instead a special, characteristic roughness (Figure 2): Systematically arranged, water-repellent, nano-size wax crystals form three-dimensional structures, similar to small nipples, which are no greater than a few nanometers or micrometers in size. When combined with the waxes' water-repellent chemical properties, these structures make the lotus leaf extremely non-wettable, a state called ultrahydrophobia or super-hydrophobia, and they give it its self-cleaning properties. Dirt particles only sit on the tip of the wax crystals and as a result only a very small surface area comes into contact with the plant's surface. If water falls onto a leaf surface like this, the interplay of the surface tension and the low attraction force between the surfaces and the water produce a spherical water drop which only sits on the tips of the wax structures. If the leaf tips in the slightest, the water drop immediately rolls off, taking the dirt particles with it (Figure 3). As the gravitational pull between the dirt and the leaf's surface is very slight, i.e. it is smaller than that between the water and the dirt, even lipophilic impurities, such as soot for example, can be washed away. In most cases this form of self-cleaning serves not so much to protect the plant from dirt as from pathogens (e.g. fungal spores, bacteria). Super-hydrophobia is a property which is not only found in the lotus plant however, it is present in around 300 other plant species as well. Insects too, such as dragonflies and butterflies have this property on the surface of their wings |
|
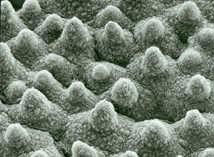 Figure 2: Electron microscope photograph of the surface of a lotus flower leaf. The combination of surface roughness and water-repellent wax cristals gives it superhydrophobic properties. The principle of self-cleaning was discovered in 1973 by the botanist Wilhelm Barthlott and his team at the University of Bonn. As it is a purely physio-chemical effect that is not bound to a living system, Barthlott believed that its technical implementation should be possible. Industry however showed little interest initially, and so he produced his own technical surfaces with these properties, applied for a patent for his invention2 and registered the brand name "Lotus-Effect®". The first commercial product made in collaboration with industry was a silicone resin house paint which has since become widely used and in which silicon nanoparticles form micro-structured surface3. Other "Lotus-Effect?"products include ceramic roof tiles, architectural glass and a spray for industrial use which makes surfaces temporarily non-wettable and self-cleaning4. Advances are also being made in the development of engineered fabrics that are selfcleaning. Composite materials made up of nanoparticles in a coating matrix make it possible to manufacture the surface structure required for the "Lotus-Effect". These polyester fabrics are of particular interest in the manufacture of awnings, parasols, sails and tents5. Their self-cleaning properties have led the Institute for Textile Technology and Process Engineering (ITV) in Denkendorf, Germany to award them the quality label "Self-cleaning inspired by nature"6. |
|
| Manufacture | |
| Manufacturing surfaces which feature a "Lotus-Effect?"is technically challenging and still poses certain problems. To date there are only a few low-cost processes which can produce the surface structures on a large scale. | |
| One example is the application of "Lotus-Effect?"structures in house paints. Also, the required surface structures can either be created from hydrophobic polymers early on at the manufacturing stage or post-production by means of moulding, etching or applying a powder made from hydrophobic polymers or from nanoparticles (e.g. silicon dioxide). A further possibility is the subsequent hydrophobisation of a previously manufactured surface which has the required structures7. | |
| Possible uses and limitations | |
| The sensitivity of these surface structures to mechanical load prevents them from being used widely. Whereas the lotus plant's structure continuously renews itself, technological imitations do not have this capability. Consequently no clothing textiles with the "Lotus-Effect" have been produced ? washing in a washing machine would destroy the surface structures. | |
 Figure 3: As it rolls off, a drop picks up the dirt particles which are lying loosely on the leaf's surface, thereby cleaning it. Self-cleaning also requires running water, rain for example, hence surface structures with these properties are not practicable for use in most interiors. Also, "Lotus-Effect"surfaces have a slightly matt finish and therefore cannot be used on optical glass (e.g. spectacles). Tensides (e.g. soap or washingup liquid) reduce the surface tension of water thereby disrupting the formation of drops. If self-cleaning surfaces are treated with detergents containing tensides, the "Lotus-Effect ?"therefore breaks down and water will make the surface wet. The surface is not destroyed by this and is fully functional again after rinsing in clear water. However, the fact of this makes "Lotus-Effect" surfaces largely unsuitable for use on ceramic sanitary facilities (e.g. washing basins, shower trays, bath tubs etc.). Several years of practical experience with house paints have shown that the self-cleaning capability is only partially successful. For example, the splash zone of the outside walls of houses (the base course) becomes dirty in spite of the "Lotus-Effect". Furthermore, it only takes a simple fingermark to remove the "Lotus-Effect"because fat cancels out super-hydrophobia8. There are still limits, therefore, to technology's ability to imitate the natural self-cleaning capability of the lotus leaf. Dirt and nanotechnology water repellent "Easy-to-Clean" surfaces |
|
| As the above demonstrates, there are still only a handful of practical applications available on the market which have the "Lotus-Effect?". Nevertheless, there are already several products with dirt-repellent and waterrepellent properties on sale which, the manufacturers claim, are based on nanotechnology or contain nanoparticles. These include, for example, ceramic sanitary facilities, spectacle lenses or textiles. Often the advertisements for these products make reference to the lotus plant, however the products do not exhibit self-cleaning properties. In other words, running water alone (rain) is not usually sufficient to clean this type of product. It is simply easier to clean since dirt does not adhere so well to the surface. Ideally, these surface coatings obviate the need for aggressive cleaning detergents. | |
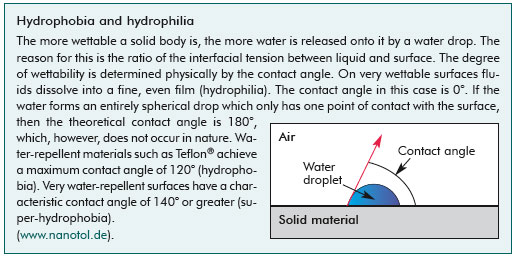 |
|
| In many cases these products have so-called "Easy-to-Clean" surfaces which, unlike products with the "Lotus-Effect", feature a smooth rather than a rough surface and are not only water-repellent but also fat-repellent (lipophobic) 9. Many commonly used materials, such as glass for example, generally have a slightly rough surface, even if they appear smooth to the eye, and attract both water and dirt. Unlike "Lotus-Effect"surfaces "Easy-to- Clean" coatings are not made rougher, but flattened by applying hydrophobic and oleophobic chemicals. However, the contact angle (see text box) between a water drop and the surface is smaller than is the case of the "Lotus-Effect" (<140°), i.e. materials treated in this way are not super-hydrophobic. Whereas the "Lotus-Effect" is based on physical as well as chemical principles, "Easyto- Clean" coatings have a purely chemical basis. Outdoor use, for example in window glass, is only practicable on particularly suitable surfaces on which sufficient water lands. Otherwise water drops may dry in places and leave dirt behind. Where there is only a very small amount of water the drops may leave behind a visible trace as they roll off9. "Easyto- Clean" does not mean that a surface with this treatment never needs to be cleaned, however the amount of cleaning required compared with that of traditional products can be reduced. Depending on the extent and nature of the dirt, a firm jet of water may suffice to remove it (e.g. in the case of glass fronts), or it may be easier to remove by mechanical means. | |
| New types of products based on chemical nanotechnology are also being used to protect the surfaces of construction materials, for example to prevent damage caused by water penetration or to protect outside walls from mildew, moss, algae and dirt. Also, protective "anti-graffiti" coatings make it easy to remove undesirable "art", as spray paints do not adhere to them. Even chewing gum can be removed more easily from surfaces coated in this way. Unlike the coatings which used to be applied for these purposes, the new silane-based products do not seal the surface, hence moisture can escape10. The protection provided is usually permanent and is retained even after repeated cleaning. Steel and glass are popular materials in architecture. However their appearance can be spoiled by, for example, fingermarks which have lipophilic characteristics. New types of "anti-fingerprint" nano-coatings counteract this as they are fat-repellent and modify the light refraction so that the marks remain invisible9. | |
| Manufacture | |
| Transparent hydrophobic and oleophobic coatings of this kind can be manufactured using sol-gel processes11. In simple terms, the raw materials are so-called silanes12 with a silicon base atom which can be modified by adding certain chemical substances to it (e.g. fluorine compounds) in order to retain the required properties. Nanoscale particles (colloids) are produced from the silanes by means of chemical reactions in a solution. The dispersion of particles is called the sol. Depending on the type of sol chosen, the solution evaporates either at room temperature or when it is heated, and the sol becomes a viscous gel because the particles concatenate into a dense web due to their high reactivity. Once it has dried out a compact layer is formed. The sol-gel process was developed as early as the 1930s, but for some years now has been ascribed to chemical nanotechnology. One of the pioneers in this field is the Leibniz Institute for New Materials (INM) in Saarbr?cken, Germany13. | |
 |
|
| Figure 4: Diagram of an "Easy-to-Clean" surface. | |
| The sol can be applied to a great variety of surfaces using traditional industrial processes such as dip coating, spraying or spin-coating. The resulting coatings are only a few nanometers thin and transparent ? an important advantage over other traditional coating materials such as Teflon?, which has a dark colour because of its graphite content. These coatings can consequently also be used on, for example, glass. Sol-gel processes are already used in the motor vehicle industry to produce water-repellent coatings for windscreens or exterior mirrors14. In industrial manufacturing processes the coating is burned into the surfaces at high temperatures, thereby increasing their durability. As a sol can also dry out at room temperature to form a firm layer, surfaces such as window glass can also be treated postproduction by the consumer or a commercial service provider (e.g. cleaning companies). A number of different liquids or sprays for this purpose are commercially available. These coatings however are not durable and need to be renewed after a certain period of time. Unfortunately, information regarding the exact constituents of post-production applications with "Easy-to-Clean" properties which can be obtained in shops or via the internet is scarce as the manufacturers often claim the right of trade secrecy. It is therefore usually impossible to establish whether products are based on chemical nanotechnology in each case. | |
| Impregnating agents are also available which make textiles and leather dirt and water- repellent and which, manufacturers claim, contain nanoparticles or are based on nanotechnology. Often, however, traditional surfactant substances are used, such as fluorocarbon resin or silicon oils, which produce a nanoscale impregnated layer. Based on the current understanding of nanotechnology, one-dimensional nanostructures would probably also be categorised as such, however it appears that many products do not fulfil an essential criterion of nanotechnology ? i.e. that the material has new properties. Nonetheless there are still impregnating materials on the market which manufacturers claim, with reference to the lotus plant, produce surface roughness and hydrophobia on materials by means of nanoparticles, making them dirt and water-repellent. | |
| Self-cleaning II – photocatalytic nanotitanium dioxide (TiO2) | |
| To date, photocatalytic self-cleaning15 is probably the most wide-spread application ascribed to nanotechnology in the construction industry. There are already a great number of buildings worldwide which have been treated with it. The photocatalytic properties of TiO2 were discovered as long ago as 1967 by Akira Fujishima, a scientist at the University of Tokyo, and the phenomenon became known as the "Honda-Fujishima Effect". The first house with self-cleaning exterior surfaces was Fujishima's own9. | |
| Titanium dioxide is hydrophilic due to its high surface energy, hence water does not form drops on a surface coated with it, but a sealed water film instead. A coating of this kind hence behaves in exactly the opposite manner to a surface whose self-cleaning properties are based on the "Lotus-Effect". It is also transparent and can therefore also be used on glass. In addition, TiO2 is photocatalytic, in other words in the presence of water, oxygen radicals are produced under UV light irradiation which in turn can decompose organic material such as, for example, fats, oils, soot or plant materials. TiO2 is especially reactive in nanoform. It is not expended during catalysis, with the result that the effect is lasting. On self-cleaning surfaces of this type organic dirt is dissolved in the water film, decomposed, and the residue is removed by the next heavy shower of rain. This does not however mean that surfaces of this kind must never be cleaned. The amount of dirt is merely reduced so that it does not need to be cleaned as often16. From an engineering point of view coating organic or polymer surfaces (e.g. plastics) with TiO2 is complicated because the carrier materials themselves, like any other organic material, are attacked and destroyed by oxidation. In order to prevent this, inorganic barrier layers need to be applied and the TiO2 nanoparticles given a surface coating in order that the different layers adhere to each other17. | |
| Photocatalytic self-cleaning only works in outdoor use because it requires UV light and water to remove the residue. Ways of modifying the properties of TiO2 are currently being researched so that it becomes photocatalytic by irradiation with visible light. This is achieved by doping them with metal atoms such as, for example, chromium, vanadium18, tungsten19 or carbon20. The results of earlier research have already been implemented in commercial products such as photocatalytically active interior paints which reduce gaseous pollutants in the air, for example to improve the air quality in enclosed office spaces21. As photocatalysis can also kill microorganisms, it is conceivable that antimicrobial coatings may be produced in the future, for example for use in clinical areas, and this is now being researched22. The ultra-thin water film formed by the hydrophilic properties of the nanoscale titanium dioxide on surfaces additionally prevents glass from misting up since water droplets cannot form. The "Anti-Fog" effect, as it is known, is particularly beneficial, for example, in glass surfaces in conservatories, exterior mirrors on motor vehicles, or even in bathroom mirrors or spectacles. | |
| Manufacture | |
| Photocatalytic coatings with nano-TiO2 are usually applied at the same time as the material itself is manufactured, using Chemical Vapor Deposition (CVD23). The coating is already in use not just in glass surfaces, but also in plastics (PVC), sound-proofing panels, tiles, roof tiles or concrete slabs. However by this method it is not possible to coat these materials post-production. In the case of glazing, it is important to ensure that silicon seals are not used as their oils seep over the glass, destroying the hydrophilic surface properties and leading to unsightly smears forming9. | |
 Figure 5: Hydrophilic coating (right) with TiO2 on float glass. As well as coating by means of CVD, sol-gel processes can also be used to create the surface functionality, for example in motor vehicle windscreens or exterior mirrors. This latter method is far more preferable for the manufacturers as it is carried out at lower temperatures, takes less time, and saves energy costs24. House paints with photocatalytically active TiO2 nanoparticles are also available, which not only have self-cleaning properties25 but which also reduce nitrogen oxide and ozone26. Environment and health Self-cleaning or easy to clean surfaces can reduce the amount of cleaning required. In the case of industrial cleaning in particular it can reduce labour costs and extend a material's durability. Lower energy costs and less use of cleaning detergents are expected to be the primary environmental benefits. It was in anticipation of such outcomes that the German Federal Foundation for the Environment (DBU) awarded Wilhelm Barthlott its respected Environment Prize in 1999 for his discovery of the "Lotus-Effect"27. However, there are currently no reliable, quantitative studies of actual potential environmental benefits. As a rule, descriptions of products' environmental benefits do not contain an analysis or evaluation of the amount of resources used and/or the energy consumption involved in their manufacture. Future evaluation should also include information on the fate and behaviour of the materials once they reach the end of their life cycle (waste stage)28. |
|
| Current risk assessments conclude that there is very little probability of a harmful impact on the environment or human health from coatings in which nanoparticles are firmly embedded in a coating matrix, as in the case of "Easy-to-Clean" coatings. There have so far also been no indications of environmental or health risks from surfaces coated with the "Lotus-Effect". A recent investigation into the abrasion resistance of test structures which had been coated with zinc oxide nanoparticle layers showed no significant release from the coating material29. It does appear possible however, that nanoparticles are released as a result of the effects of weathering on the coating matrix, for example where they consist of biodegradable materials. An investigation by Kaegi et al. has shown that house paints release very small amounts of synthetic TiO2 particles, between 20 and 300 nm in size, as a consequence of weathering, and that these can enter the soil via rainwater drains30. The photocatalytic activities of TiO2 produce free oxygen radicals that are toxic for aquatic organisms if such nanoparticles enter the waters they inhabit31. To date, threshold levels remain unknown. The release of particles into the environment can however be reduced or prevented if nanocoatings and nanomaterials are designed accordingly32. | |
| Although surface coatings which have nanomaterials firmly embedded in a matrix are currently believed to pose only a very slight risk to the health of users and consumers, the protection of those who work in the companies which manufacture nanoparticulate raw materials requires special attention. The German State of Hesse's Ministry for Economic Affairs, Transport and State Development recently commissioned a study by the Institute for Applied Ecology in Darmstadt (?ko-Institut Darmstadt) which confirmed in its action recommendations for the manufacture and use of nanomaterials in the paints and coatings industry that there are significant gaps in knowledge and information in respect of exposure data and human and eco-toxicological impact. The report goes on to recommend that companies should be guided by the precautionary principle and that preventing inhalation be given top priority. Where this proves impossible, appropriate effective protective measures must be implemented (e.g. filters, extraction units, respiratory masks etc.). The release of particles into the environment must be kept to the minimum possible33. | |
| Impregnating agents in propellant sprays can pose a danger to consumers' health if not properly used. This is independent, however, of whether they contain traditional surfactant substances or are, according to the manufacturers, based on nanotechnology. In 2006 approximately just over 100 people had to have medical treatment for serious respiratory difficulties after they had used a so-called "nano-spray" to treat glass and ceramic surfaces in bathrooms. The cause was initially suspected to be nanoparticles. However, chemical analyses showed that the products did not contain nanoparticles. "Nano" in the product descriptions referred only to the nanoscale protective layer produced by the spray. The probable cause of the health problems was the combination of the surfactant substances (fluorosilanes) with the spray's other chemical components34. Propellant sprays produce aerosols (tiny liquid droplets) between a few dozen nanometers to around a hundred micrometers in size. If inhaled, the larger droplets can become blocked in the nose and the upper part of the respiratory tract. However, droplets smaller than 10 micrometers can penetrate deep into the lung, in certain circumstances as far as the alveoli, which may collapse as result of the effect of the surfactant substances from the impregnating sprays35. Inhaling the atomised spray from these types of products must therefore be avoided at all costs and their directions for use must be adhered to precisely. Use in enclosed spaces (such as a bathroom) is inadvisable under any circumstances. Manufacturers should adjust spray systems to ensure that droplets no smaller than 10 micrometers cannot be sprayed. As a general rule, it is more advisable to use impregnating agents ? whether advertised as "nano" or not ? which come in manual atomisers without propellants (pump sprays) or in liquid form (e.g. as a foam). | |
| Notes and References | |
| 1 Veith, M. and Schubert, M., 2009, Innovativ und Smart ? Funktionale Oberfl?chen schaffen hohen Nutzen, Lippe Wissen & Wirtschaft, 4, 34-35. | |
| 2 European Patent EP 0772514, 15.2.96, Selfcleansing surfaces of objects and methods for the production thereof. | |
| 3 www.sto.de, 24.3.10. | |
| 4 www.degussa-household-care.com, 24.3.10. | |
| 5 www.basf.de/science_around_us, 24.3.10. | |
| 6 www.itv-denkendorf.de, 24.3.10. | |
| 7 European Patent EP 0772514 (Endnote 2). Ramaratnam, K. et al., 2008, Ultrahydrophobic Textiles Using Nanoparticles: Lotus Approach, Journal of Engineered Fibres and Fabrics 3(4) | |
| 8 www.maler-kempf.de/html/thema_lotusan.html, 4.3.10. | |
| 9 Leydecker, S., 2008, Nanomaterialien in Architektur, Innenarchitektur und Design: Birkh?user Verlag. | |
| 10 www.protectosil.com, 25.3.10. | |
| 11 See NanoTrust Dossier 006, epub.oeaw.ac.at/ita/nanotrust-dossiers/dossier006.pdf. | |
| 12 The term silane refers to a group of chemical compounds comprising a basic silicon frame and hydrogen. | |
| 13 www.inm-gmbh.de. | |
| 14 Aegerter, M.A., et al., 2008, Coatings made by sol-gel and chemical nanotechnology, J Sol-Gel Sci technol (47), 203-236. | |
| 15 Photocatalytic self-cleaning is the property of surfaces coated with titanium dioxide (TiO2) nanoparticles. Titanium dioxide (TiO2) is a semiconductor; electron hole pairs generate light if the energy of the photons is greater than the band gap Eg (inner photoelectrical effect). The electrons or holes can diffuse to the surface in titanium dioxide and produce radicals that break down organic substances. The holes in particular have a strong oxidising effect; OH radicals are produced from water, as organic substances are broken down. The end products are often CO2 and water. The band gap Eg is .2 eV for anatas, the form of TiO2 that is most efficient for photocatalysis (ca. 3.0 eV for the less efficient rutil crystal structure). Since this energy corresponds to a wavelength of ca. 390 nm, only ultraviolet light is effective. The super-hydrophilic properties of the surfaces are created by oxygen gaps on the TiO2 surface. It is here that the OH groups are bonded to allow a good netting with water. | |
| 16 Fujishima, A. and Zhang, X., 2006, Titanium dioxide photocatalysis: present situation and future approaches, Comptes Rendus Chimie 9 (5-6), 750-760. | |
| 17 Quilitz, M. et al., 2008, Innovative Schichtsysteme ? Neue Entwicklungen der chemischen Nanotechnologie, Journal f?r Oberfl?chentechnik 7, 8-10. | |
| 18 Anpo, M., 2000, Utilization of TiO2 photocatalysts in green chemistry, Pure Appl. Chem. 72(7), 1265-1270 | |
| 19 Lorret, O. et al., 2009, W-doped titania nanoparticles for UV and visible-light photocatalytic reactions, Applied Catalysis B: Environmental 91, 39-46. | |
| 20 Sakthivel, S. and Kisch, H., 2003, Tageslicht-Photokatalyse durch Kohlenstoff-modifiziertes Titandioxid, Angewandte Chemie 115, 5057-5060. | |
| 21 www.sto.de/evo/web/sto/27762_DEInnovationen-StoClimasan_Color.htm, 10.6.10. Yu, J. C., Ho, W., Yu, J., Yip, H., Wong, P. K. and Zhao, J., 2005, Efficient Visible-Light-Induced Photocatalytic Disinfection on Sulfur-Doped Nanocrystalline Titania, Environmental Science & Technology 2005(39), 1175-1179. | |
| 22 In chemical vapour deposition (CVD) at least two volatile compounds are fed with a gas flow into a reaction chamber containing the object to be coated. The addition of reaction energy causes a chemical reaction to take place on the surface of the object. If the displacement of a layer on the surface is to be preferred to the formation of solid particles in the gas phase, the CVD must take place at very low pressures. | |
| 23 Aegerter, M.A. et al., 2008, Coatings made by sol-gel and chemical nanotechnology, J Sol- Gel Sci technol (47), 203-236. | |
| 24 www.nanprom.de/fassadenfarbe.htm, 24.3.10. | |
| 25 stophotosan-nox.de, 24.3.10. | |
| 26 www.dbu.de/575.html, 10.6.10. | |
| 27 Becker, H. et al., 2009, Nanotechnik f?r Mensch und Umwelt ? Chancen f?rdern und Risiken mindern; Umweltbundesamt Dessau | |
| 28 Vorbau, M. et al., 2009, Method for the characterization of the abrasion induced nanoparticle release into air from surface coatings, Aerosol Science 40, 209-217. | |
| 29 Kaegi, R. et al., 2008, Synthetic TiO2 nanoparticle emission from exterior facades into the aquatic environment, Environmental Pollution 156, 233-239. | |
| 30 Battin, T.J., et al., 2009, Nanostructured TiO2: Transport Behavior and Effects on Aquatic Microbial Communities under Environmental Conditions, Environmental Science & Technology 43(21), 8098-8104. | |
| 31 K?lbel, M., 2008, Steckt der Teufel im Teilchen? ?ber die Risiken der Nanotechnologie, in: S. Leydecker (Endnote 9), 44-49. | |
| 32 Hermann, A. et al., 2009, Sichere Verwendung von Nanomaterialien in der Lack- und Farbenbranche ? Ein Betriebsleitfaden, commission by Verkehr und Landesentwicklung Hessisches Ministerium f?r Wirtschaft: HA Hessen Agentur GmbH www.hessen-nanotech.de/dynasite.cfm?dsmid=5374. | |
| 33 2nd Session of the BfR commission "Bewertung von Vergiftungen", minutes dated 27./28.4.09. | |
| 34 "Treibgassprays: ein Gesundheitsrisiko?" Bundesamt f?r Gesundheit (BAG) Schweiz, 12/07. | |
| 35 Muntwyler, R., 2004, Nicht nur Leder, auch die Lunge wird impr?gniert, K-Tipp, 16-17 www.ktipp.ch/downloadfile/1020525. | |
| By NanoTrust, Austrian Academy of Sciences. NanoTrust Dossiers are published irregularly and contain the research results of the Institute of Technology Assessment in the framework of its research project NanoTrust. The Dossiers are made available to the public exclusively on epub.oeaw.ac.at/ita/nanotrust-dossiers. | |
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
