| Posted: Apr 29, 2013 | |
Porphysomes may enable a radically different approach to treating prostate cancer |
|
| (Nanowerk Spotlight) Men diagnosed with prostate cancer are often treated with a ‘radical’ prostatectomy, a procedure that involves removing the entire prostate gland. While this treatment is usually effective at preventing the spread of the disease, it can lead to complications. For many men, radical therapy ‘over-treats’ prostate cancer since only a small part of the prostate contains cancerous cells. ‘Focal therapy’, in contrast to radical therapy, involves removing or ablating only the part of the prostate that is cancerous. However, it is often difficult for doctors to distinguish cancerous tissue from surrounding healthy tissue. | |
| Reporting in ACS Nano ("Inherently Multimodal Nanoparticle-Driven Tracking and Real-Time Delineation of Orthotopic Prostate Tumors and Micrometastases"), a team of researchers at the University of Toronto and the University Health Network used a new type of biocompatible nanoparticle known as a ‘porphysome’ to detect and demarcate prostate tumors as small as 2mm in size. The tumors were imaged using a combination of positron emission tomography (PET) and optical imaging. PET allows doctors to detect, localize, and characterize prostate tumors non-invasively, while optical imaging allows them to find the edges of the tumor(s) during treatment. The porphysomes were also able to detect cancerous cells that had metastasized (spread) to the bone. This may allow doctors to find and treat cancer that has spread from the prostate to other parts of the body. | |
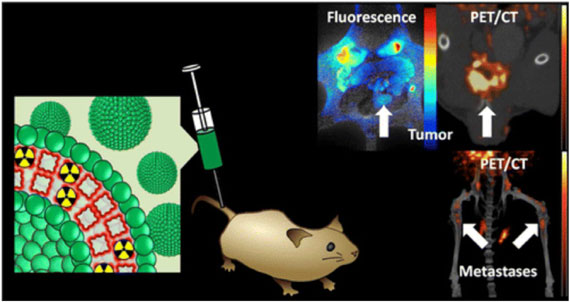 |
|
| Radio-labeled porphysomes for combined positron emission tomography (PET) and optical (fluorescence) imaging of cancer. (Figure adapted with permission. ©2013, American Chemical Society) | |
| Focal therapy creates fewer side effects than radical therapy, yet it comes with an attendant risk. If some of the cancerous cells are left behind, the tumor may grow back, or worse, metastasize (spread) to other parts of the body. For effective focal therapy, doctors need a way to ‘see’ the tumor(s) within the prostate gland using clinical imaging techniques, so that they can be removed completely. Ideally, prostate tumors would be first imaged ‘non-invasively’. Non-invasive imaging allows the tumor to be seen from outside of the body without having to perform surgery. This initial imaging step would allow doctors to find the location of the tumor(s) within the prostate, their size, and whether the disease has spread to surrounding tissues. Then, a complementary imaging technique would allow visualization of the boundaries of the tumor (known as the ‘margins’) during surgery. Identifying the margins of the tumors helps surgeons to remove all of the cancerous cells, ensuring that none are left behind. | |
| As a step towards addressing this need, Dr. Gang Zheng and his colleagues developed a radio-isotope-labeled porphysome nanoparticle that could simultaneously image prostate cancer using two different clinical imaging techniques: positron emission tomography (PET) and optical imaging. Porphysomes, which were pioneered by Dr. Zheng’s group, are formed by the self-assembly of ‘porpholipids’ into spherical nanostructures. Porpholipids are formed by chemically-fusing porphyrin molecules with phospholipids. When the porpholipid is suspended in water and heated, it spontaneously assembles into bilayers, or sheets of the lipids. These bilayers can then be extruded through a tiny perforated membrane to form balloon-like nanoparticles that are ~100nm in diameter (see schematic). | |
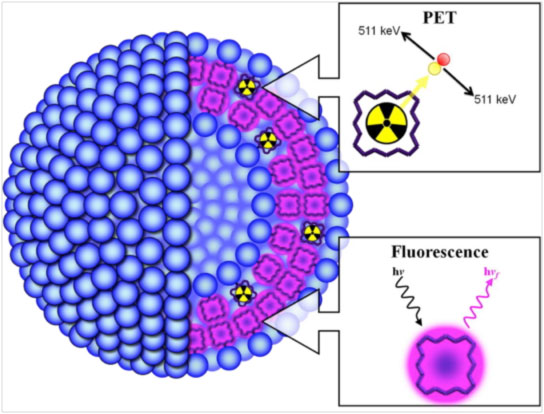 |
|
| Schematic showing the structure of porphysomes formed by the self-assembly of porpholipids. Porphyrin in the porpholipids in naturally fluorescent. Porphyrin can also chelate radioisotopes so they can be detected by positron emission tomography (PET). (Figure adapted with permission. ©2013, American Chemical Society) | |
| Zheng explains that porphysomes are fundamentally different from porphyrin-loaded liposomes in at least three ways: loading capacity, stability, and optical properties. “Porphyrins are bulky molecules. You can’t load them in liposomes beyond about 5-10% until the structure is disrupted. But, you can pack about 80,000 porphyrin molecules in a single porphysome... On top of that, porphysomes are more stable than liposomes. Porphyrins within the porphysome can interact with each other through their aromatic rings. This leads to more structural stability... Finally, porphysomes also be used as photothermal agents. By combining so many porphyrin molecules together, porphysomes have extinction coefficients on the order of 1010, which rivals gold nanoparticles. This property gives porphysomes a photothermal effect that can be used for therapy and for photoacoustic imaging.” | |
| Porphyrin molecules are naturally fluorescent. Exciting them with light at one wavelength (~410nm) leads to the re-emission of light of a higher wavelength (~600nm). The emitted light can be detected using a charge-coupled device, similar to those in digital cameras. By accumulating selectively in prostate tumors, porphysomes create an optical signal that surgeons can use to see tumor margins during surgery. Seeing the margins allows them to either remove or ablate the tumors, while sparing the surrounding tissue. In addition to their natural fluorescence properties, porphyrin molecules also have a small chemical ‘cavity’ in its center that can ‘chelate’ radioactive metal ions, like 64Cu. As it decays, 64Cu emits gamma radiation that can be detected by a PET scanner. By ‘doping’ some of the porpholipids with 64Cu2+, Zheng and his team created porphysomes that could be visualized simultaneously using both PET and optical imaging (see schematic). | |
| Zheng explains that the strength of the porphysome for imaging prostate tumors lies in its ability to combine both PET and optical imaging modalities together into a single nanoparticle. “The beauty of PET and fluorescence is that they are complementary techniques providing different kinds of information. The sensitivity of PET is so high, and there’s almost no attenuation in tissues. Fluorescence, on the other hand, is sensitive and easy to detect, but it doesn’t penetrate deep into tissues. It’s great for imaging during surgery because it’s fast and simple.” He goes on to explain that within a porphysome, it is vital that only a small fraction of the porpholipids are modified with 64Cu2+. “ Chelating 64Cu2+ quenches the porphyrin fluorescence. By only modifying a fraction of the porpholipids, you still retain the overall fluorescence of the construct.” | |
| The researchers tested how well the radiolabeled porphysomes could detect prostate tumors grown ‘orthotopically’ in mice. Orthotopic tumors are human prostate cancer cells that are grown within the prostate glands of mice, and are relevant to human prostate cancer. In the first 4 hours after injecting porphysomes into the blood of these mice, the tumors showed little contrast by PET (see image). At this time-point, porphysomes were still present in the blood and surrounding healthy tissues. However, after 24 hours, the tumor could be clearly distinguished from surrounding healthy tissues (see image). When the researchers performed a mock surgery, they could clearly see the areas of the prostate that had been invaded by cancerous cells by using optical imaging (see image). The researchers found that they could detect tumors as small as 2mm in diameter, showing that porphysomes could potentially be used to detect prostate cancer at the earliest stages of its onset. | |
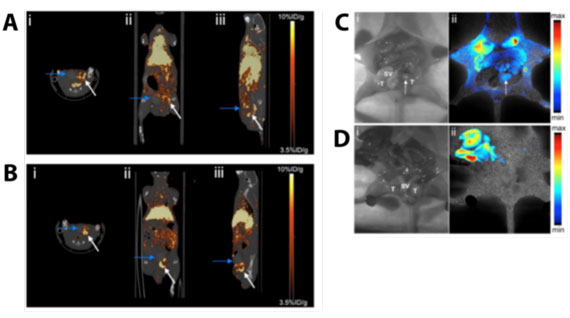 |
|
| Positron emission tomography (PET) and optical imaging of prostate cancer tumors in mice. PET imaging of tumors (A) 0h, and (B) 4h after injection of porphysomes. Arrows show location of the prostate. Fluorescence imaging of mice with (C) or without (D) prostate tumors. (Figure adapted with permission. ©2013, American Chemical Society) | |
| Emboldened by the sensitivity with which porphysomes could detect prostate cancer tumors, Zheng and his team examined whether the particles could also detect metastases. Metastases are small ‘pockets’ of cancer cells that have spread beyond the organ where they originated (in this case the prostate) and invaded other parts of the body. Metastases are small and widely-distributed in the body, making them hard to detect by conventional imaging, and even harder to treat. They are one of the major causes of cancer-related death. Prostate cancer, if left untreated, often spreads to surrounding organs like the lymph nodes, bones, and spinal cord. At that point, even radical prostatectomy does not cure the disease. Using a metastatic model for cancer established in mice, Zheng and his colleagues observed metastases as small as 2mm in diameter in the bones and spinal column of mice. This is one of the first demonstrations of a nanomaterial that is able to selectively detect bone metastases. | |
| One of the advantages of porphysomes over other nanoparticle types is their versatility. In addition to modifying porphysomes with 64Cu2+, Zheng and his colleagues believe that they could also load them with therapeutic radioisotopes. Therapeutic radioisotopes emit a type of radiation known as ‘beta particles’, which can damage tissues. If porphysomes loaded with these radioisotopes accumulate selectively in prostate cancer tumors, they would be able to deliver radiation specifically to the cancerous tissues, while avoiding healthy tissues. | |
| “The beauty of the porphysome is that we’re not limited to using a radiotracer. If we switch the imaging radioisotope to another therapeutic radioisotope, we can actually start to think about not just imaging tumors but also treating them.” Zheng goes on to explain the advantage of the radioisotope strategy over alternatives like photodynamic therapy and photothermal therapy. “If we wanted to use photodynamic therapy or photothermal therapy, we could only treat a location that is accessible to light. Light can’t be delivered anywhere you want it to be. There are issues with tissue penetration. If you loaded them with a therapeutic radioisotope like 67Cu or 177Lu, you could use the porphysome to treat the tumors directly. The advantage of this type of treatment is that there is no need for surgery.” | |
| While the authors focused their efforts in this study on prostate cancer, Zheng believes that porphysomes have the potential to treat many different types of cancer. “The general principle could be adapted to treating a number of different cancer types, including ovarian, lung, or even pancreatic cancer.” To help direct porphysomes to other tumor types Zheng points out that they could be modified with ‘targeting ligands’ that helps direct them to different cancer types. “By swapping targeting ligands, the same basic porphysome structure could be made specific for multiple different types of cancers.” | |
| The encouraging results shown by Zheng and his team in this study suggest that porphysomes may be a viable in human patients. On top of the modularity in their design, another advantage that porphysomes have over other nanoparticle platforms is that they are naturally-derived and biocompatible. Porphyrin itself is harvested from algae. Porphysomes can be completely degraded and cleared within the body, so there is little risk of toxicity. In a previous study, Zheng and his colleagues showed that porphysomes did not produce any adverse effects in mice. In contrast, inorganic nanomaterials made from metals, semiconductors, and silica tend to accumulate in the body, raising the potential for long-term toxicity, and creating a barrier to their clinical translation. Porphysomes can also take advantage of the manufacturing platform that is already in place for producing liposomes for clinical applications. | |
| Although porphysomes are not ready for use in human patients quite yet, their impressive performance, biocompatibility, and manufacturing advantages means that they could be one of the newest of the next generation of medical nanomaterials to enter clinical use. Far from being just an incremental improvement over existing technologies, it is likely that porphysomes will be revolutionary in the fight against not just prostate cancer, but cancers in general. | |
| By Carl Walkey, Integrated Nanotechnology & Biomedical Sciences Laboratory, University of Toronto, Canada. | |
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
