| Posted: Jun 19, 2013 | |
Atomic resolution images show what happens when lithium ions enter battery electrodes |
|
| (Nanowerk Spotlight) Lithium-ion batteries are some of the most energetic rechargeable batteries available today – thanks to their good energy-to-weight ratios – and consequently, they power most of the electronic devices that we carry around with us. | |
| In terms of weight and size, batteries have become one of the limiting factors in the continuous process of developing smaller and higher performance electronic devices. To meet the demand for batteries having higher energy density and improved cycle characteristics, researchers have been making tremendous efforts to develop new electrode materials or design new structures of electrode materials. Just recently, for instance, scientists have dramatically improved the performance of lithium-ion batteries by creating novel electrodes made of silicon and conducting polymer hydrogel, a spongy material similar to that used in contact lenses and other household products (read more: "Novel nanotechnology electrodes that improve lithium-ion batteries"). Other novel electrode materials are silicon and boron (see: "Promising material for lithium-ion batteries"), and of course carbon nanomaterials such as graphene (see: "Graphene improves both energy capacity and charge rate in rechargeable batteries"). | |
| "The design of new electrode materials to a great extent depends on how the lithiation front propagates into the anode material," Reza Shahbazian-Yassar, an Associate Professor at Michigan Technological University (MTU) and a Visiting Associate Professor at the University of Illinois at Chicago, tells Nanowerk. "Therefore, revealing the atomic-scale lithiation mechanism is central to unfolding the performance of electrode materials during the operation of lithium-ion batteries." | |
| In new work, reported in the June 3, 2013 online edition of ACS NANO ("Atomic-Scale Observation of Lithiation Reaction Front in Nanoscale SnO2 Materials"), Yassar and his team investigated the atomistic nature of the lithiation mechanism in individual tin dioxide (SnO2) nanowires by in situ transmission electron microscope (TEM) and complementary density functional theory (DFT) simulation. | |
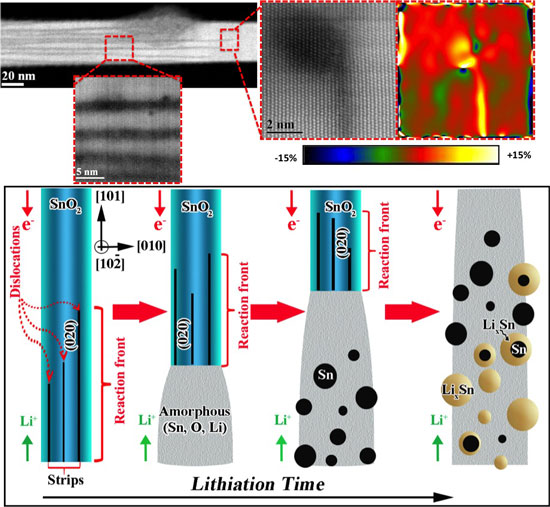 |
|
| "(top) High angle annular dark field imaging of the lithiation reaction front in a SnO2 nanowire. The atomic resolution images show the parallel Li-ion intercalation channels and the formation of dislocations at the tip of the channels. The straining at the tip of the channels was estimated to be ~ 15%. (below) Schematic of the lithiation characteristics of the crystalline SnO2 nanowire featuring the initial lithium-ion long-range diffusion mediated by dislocation activities inside the pristine lattice, followed by solid-state amorphization of the crystalline nanowire, nucleation of Sn particles, and finally the alloying of the Sn particles into LixSn. (Image: Dr. Reza Shahbazian-Yassar, Michigan Technological University) | |
| "Our work provides an interesting insight into the initial stages of lithiation in SnO2 materials," Yassar explains. "The general understanding in the Li-ion battery community is that, as Li-ions move into crystalline SnO2, a solid-state amorphization happens that eventually leads to the formation of Li-Sn alloys. However, we have shown that this understanding is not quite accurate. In fact, as Li ions move into the SnO2 lattice, they prefer to move (or intercalate) along particulate crystal planes/directions as the lattice energy expansion is in favor of their long-range intercalation." | |
| In their paper, the team, which in addition to MTU researchers included scientists from the University of Illinois at Chicago, King Abdullah University of Science & Technology (KAUST) in Saudi Arabia, Zhejiang University in China, shows that this long-range intercalation process is mediated with the formation of dislocations that results in straining the SnO2 lattice up to 15%. This long-range intercalation then is followed with solid-state amorphization with the addition of more lithium. This explains why the crystallographic directions of the battery electrodes can be very important for battery operation. | |
| The findings of this study provide important insight into the physical basis of microstructural evolution, morphological changes, and mechanical degradation in SnO2 electrodes. | |
| As Yassar points out, this is the first atomic resolution imaging of the lithiation reaction front in any crystalline materials. | |
| "We were puzzled with the question of what happens when lithium ions enter a crystalline lattice," he describes the motivation behind this work. "We were not convinced with the general belief that the solid-state amorphization happens instantly. Something else must have triggered this amorphization. Fortunately, we had access to one of the highest resolution electron microscopes in the United States allowing us to resolved interatomic spaces with resolutions better than 0.7 Å. That's why we decided to give this a shot." | |
| This work is directly relevant to the current research and development of Li-ion batteries. It shows that the engineering of crystallographic orientation and interatomic spacing can play a significant role in the performance of battery electrodes. | |
| Building on this work, the researchers want to study the structural evolutions during delithiation. "We want to know what intermediate structural changes happens as lithium ions start to leave the host electrode," says Yassar. "A question of interests would be: is the observed microstructural transitions reversible?" | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
