| Posted: Jul 30, 2013 | |
Release of silver from nanotechnology-based products for children |
|
| (Nanowerk Spotlight) Not counting the sometimes alarmist reports from environmental and consumer groups (see for instance: "Groups file legal action for EPA to stop sale of 200+ nanosilver products"), the ongoing discussion among scientists about the potential environmental and health effects caused by silver nanoparticles in consumer products ranges from the cautionary (see for instance: "Silver nanoparticles used in consumer products may adversely affect environment") to the mollifying (see: "Barely any nanosilver from consumer products in the water"). | |
| Contributing to an incomplete and confusing picture, in the literature, silver nanoparticles are claimed as nontoxic or toxic depending on their size, concentration and surface functionalization (read more: "Surface chemistry of silver nanoparticles and cell death"). | |
| Assessing the potential effects of nanomaterials on environment and human health consists of two distinct aspects: To what degree are nanoparticles released from products; and how and to what degree do the released nanoparticles affect organisms? | |
| The first aspect is centered on a field called exposure science, the study of human contact to agents – such as chemicals or microbes – found in their surroundings. With regard to nanotechnology, exposure scientists are addressing a knowledge gap between the toxicity of nanoparticles to humans and the popularity of consumer products that contain nanomaterials in the marketplace. | |
| "We know that many consumer products contain nanomaterials and the safety of those nanomaterials has not been proven yet," Marina Quadros tells Nanowerk. "Therefore we would like to know how much of a certain nanomaterial a consumer may be exposed to during the normal, real-world use of consumer products." | |
| A new paper in Environmental Science & Technology, first-authored by Quadros, an associate director for Virginia Tech's Center for Sustainable Nanotechnology (VTSuN), looks at the release of nanosilver from consumer products for children. | |
| "Our core finding is that the release of silver from nanosilver-containing products depends heavily on how the product is used," says Quadros. "The total amount of silver released by a consumer product is likely to be very low and, for the products tested, happened only in the beginning of product life." | |
| These findings support the more theoretical studies in silver dissolution. | |
| The research project, which was conducted by scientists from VTSuN, the U.S. Environmental Protection Agency (EPA), and the U.S. Consumer Product Safety Commission (CPSC), was initiated in response to the CPSC's interest in developing reliable methods for quantifying and characterizing silver release from children’s consumer products. | |
| "We were motivated by the popularity of consumer products advertising to contain silver nanotechnology, many of which are meant to be used by and around children, and we wanted to assess realistic exposure from real consumer products during scenarios of realistic use," notes Quadros. | |
| The team compiled an inventory of 82 consumer products that were claimed by the manufacturer to contain nanosilver or silver and that may be used by or around children. From this list, they selected 13 products for testing, including one plush toy (teddy bear), three fabric products (baby blanket, sleepsuit, pair of baby scratch mitts), one set of breast milk storage bags, two sippy cups, three cleaning products (disinfecting spray, surface wipe, kitchen scrubber), two humidifiers claiming to contain silver to prevent biofilm formation in the water tank, and one humidifier accessory (a cube that can be placed in a humidifier’s reservoir). | |
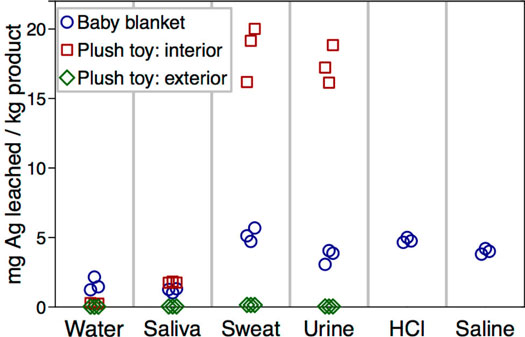 |
|
| Amount of silver released into different leaching media (all data points shown). Data points are slightly offset to improve legibility. (Reprinted with permission from American Chemical Society) | |
| "We analyzed these 13 items for their potential to release silver under conditions of normal, real-world use, defining release scenarios based on each product’s intended use and whether it might come into contact with liquids (water, milk, saliva, sweat, or urine), touch the skin, or release silver-containing aerosols," explains Quadros. | |
| She points out that bioavailability of nanomaterials in environmental studies is a concept that does not yet have standard testing methods. | |
| In their experiments, the researchers assessed the bioavailability of silver to children by considering the concentration, size distribution, and location of silver within products, as well as whether silver is released from the products into air, synthetic biological liquids, or onto skin. | |
| The team concludes that the levels of silver to which children may potentially be exposed during the normal use of these consumer products is predicted to be low, and bioavailable silver is expected to be in ionic rather than particulate form. According to them, a likely scenario is that areas of the fabric product that are exposed to sweat and urine will release small amounts of silver – < ∼5% for the products tested in this study – until the exposed silver particles become coated in silver chloride and cease releasing silver ions. | |
| Putting their findings into perspective by comparing them to animal studies, they conclude that "the quantity of silver to which children would maximally be exposed is lower than the scaled dose at which slight liver damage would be expected." | |
| Quadros says that silver – nanoparticles or ions – at low levels has not been proved to cause health problems. "However, the possibility for consumers to become chronically exposed to silver from multiple sources will be minimized if all products that use silver advertise their use and the amounts used. By measuring the amount of nanosilver in many different consumer products, we can educate consumers concerning the nanosilver ingredients that are present in the product and what their likely exposure might be due to typical use." | |
| She adds that more exposure studies on realistic, real-world scenarios would be useful to the scientific community. "These types of studies are extremely challenging to conduct because of the many different chemical ingredients found in consumer products in a home. But advancements in our analytical instruments, combined with smart sampling methods are making that possible." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
