| Posted: Oct 10, 2014 | |
CNT@NCNT coaxial nanocables - Toward full exposure of 'active sites' |
|
| (Nanowerk Spotlight) The key electrode reactions for renewable and high-capacity energy systems like fuel cells and metal-air batteries are multi-electron processes called oxygen reduction reaction (ORR) and oxygen evolution reaction (OER). The performance of these reactions is is substantially affected by the activity of the catalyst used for the electrode material. | |
| In spite of high catalytic activity, conventional noble metal catalyst materials like platinum, ruthenium, and iridium all suffer from their high cost and poor stability. As a result, scientists are seeking for substitute catalysts from non-noble metal and even non-metal materials. One solution could be found in nanocarbon materials, which afford much improved reactivity and catalytic performance. | |
| In previous Nanowerk Spotlights (see for instance: "Novel nanocarbon architecture makes a superior bifunctional electrocatalyst" or "Aligned carbon nanotube/graphene sandwiches for high-rate lithium-sulfur batteries") we have reported on the research on carbon nanomaterials for energy systems by the groups of professors Qiang Zhang and Fei Wei at Tsinghua University in Beijing. In new work, Zhang and Wei, together with Dang-Sheng Su, a professor at the Institute of Metal Research, Chinese Academy of Sciences, have now demonstrated a unique coaxial carbon nanocable material with pristine carbon nanotubes (CNTs) as the core and nitrogen-doped wrinkled carbon layer as the shell. | |
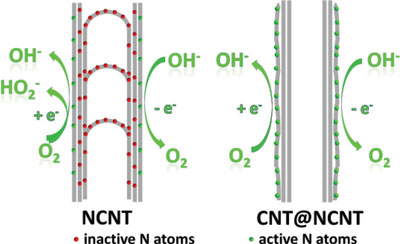
| As the team reports in the October 15, 2014 edition of Advanced Functional Materials ("Toward Full Exposure of 'Active Sites': Nanocarbon Electrocatalyst with Surface Enriched Nitrogen for Superior Oxygen Reduction and Evolution Reactivity"), the surface of these coaxial nanocables (CNT@NCNT) is enriched with N dopant atoms, that is, the N-incorporation induced active sites are fully exposed at the surface. | |
| "The incorporation of nitrogen atoms into the carbon frameworks can effectively modulate the electronic structure of the surrounding carbon atoms and tune the local charge density distribution, which results in the improvement of the chemical reactivity and subsequently promotes the catalytic performance," Zhang explains to Nanowerk. "However, in most bulk-doped nitrogen-doped carbon nanotubes the nitrogen atoms distribute uniformly, i.e. also on the inner – and thus barely accessible – nanotube walls." | |
| By contrast, the active sites rendered by the surface enriched dopant atoms on the carbon nanocables are accessible and effective to catalyze the oxygen involved electrochemical reactions. Therefore, the as-obtained CNT@NCNT nanocables afforded higher ORR/OER current compared with the routine bulk doped nitrogen-doped carbon nanotubes (NCNT). | |
 |
|
| N-doped coaxial carbon nanocables with active sites effectively exposed at the surface for oxygen reduction and evolution reaction. (Image: Department of Chemical Engineering, Tsinghua University) | |
| To fabricate the CNT@NCNT coaxial nanocables, Gui-Li Tian, a graduate student and the first author of the paper, developed a facile non-liquid phase method. | |
| "A thin N-containing turbostratic – a crystal structure in which basal planes have slipped out of alignment – carbon layer can epitaxial grow on the outer walls of pristine CNTs by CVD of N-containing compounds, resulting in the coaxial nanocables constituted by the cylindrical CNT walls and the wrinkled N-doped layers," explains Tian. "The dopant N atoms are enriched at the surface of the as-fabricated nanocables. And the inner walls remained intact as expected, leading to a high electrical conductivity of 3.3 S cm-1." | |
| As the researchers points out, combining both the merits of surface-enriched dopant N atoms and continuous inner walls, CNT@NCNT possesses superior electrocatalytic activity. | |
| "Compared with the routine bulk-doped NCNTs at similar doping level, the CNT@NCNT catalyst afforded higher current density and lower overpotential both for oxygen reduction and evolution reaction," adds Wei. "Not only are the active surface sites induced by the doping atoms more accessible to reactants, the polarity and hydrophily of the carbon material are also improved which facilitated mass transfer at the interface between electrode material and electrolyte." | |
| He also notes that, in addition, high electrical conductivity attributed to the intact inner walls benefit rapid charge transfer from the N-doped layers into the CNT scaffolds. "As a result, CNT@NCNT affords superior electrocatalytic performance in comparison with routine NCNTs." | |
| Su points out that, in addition to superior catalysts for oxygen electrochemistry, CNT@NCNT coaxial nanocables are also a good platform towards full exposure of active sites for robust interfaces in high performance composites, as well as efficient catalysts and/or metal nanoparticle supports for selective oxidation reaction and biosensors, etc. | |
| Since the surface hetero-junction nanostructures are not limited to CNTs, the researchers foresee a new branch of chemistry evolving in the area of full exposure of active sites through the 3D heterogeneous systems. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
