| Posted: Oct 17, 2014 | |
Carbon-nanotube paper electrodes with very high loading for lithium-sulfur batteries |
|
| (Nanowerk Spotlight) Today's electric vehicles, renewable energy storage, and many electronic gadgets are typically powered by lithium-ion batteries. The chemistry of lithium-ion batteries, however, limits how much energy they can store and this mature technology has reached its theoretical limit. | |
| One promising alternative is the lithium-sulfur battery, which theoretically can hold as much as four times (2600 Wh kg-1) more energy per mass than lithium-ion batteries. The downside of lithium-sulfur batteries is that they have a much shorter lifespan because they can't currently be charged as many times as lithium-ion batteries. | |
| The abundance and environmentally friendly nature of the element sulfur as cathode material contributes to the huge potential of lithium-sulfur batteries. Researchers have already shown that a combination of nanocarbon and sulfur is an effective routine to overcome the insulating nature of sulfur for lithium sulfur batteries (read more: "Aligned carbon nanotube/graphene sandwiches for high-rate lithium-sulfur batteries" and "Nitrogen tunes carbon-sulfur interfaces for stable lithium-sulfur batteries"). | |
| "Due to excellent electrical conductivity, mechanical strength and chemical stability, nanocarbon materials have been playing an essential role in the area of advanced energy storage," Dr. Qiang Zhang, an associate professor at the Department of Chemical Engineering at Tsinghua University, tells Nanowerk. "However, most contributions concerning carbon/sulfur composite cathodes possessed a relatively low areal loading of sulfur of less than 2.0 mg cm-2, which prevents the full demonstration of the outstanding performance of C/S composite cathodes. | |
| "The areal capacity of commercially used lithium-ion batteries is about 4 mAh cm-2, and therefore, the areal loading of sulfur in the cathode of lithium-sulfur batteries needs to be greatly improved," adds Qiang. | |
| Zhang and his collaborators have now created a free-standing carbon nanotube paper electrode with high sulfur loading for lithium-sulfur batteries. As the team reports in Advanced Functional Materials ("Hierarchical Free-Standing Carbon-Nanotube Paper Electrodes with Ultrahigh Sulfur-Loading for Lithium–Sulfur Batteries"), they employed a bottom-up strategy to design and fabricate a hierarchical structure. | |
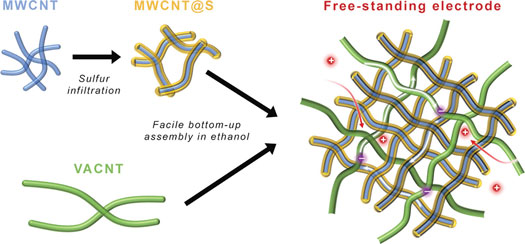 |
|
| Schematic illustration of the hierarchical, free-standing electrode with ultrahigh sulfur-loading capability via a facile bottom–up approach. Red and purple spheres represent lithium ions and electrons, respectively. (Reprinted with permission by Wiley-VCH Verlag) | |
| The team selected carbon nanotubes (CNTs) – one of the most efficient and effective conductive fillers for electrodes – as the building block. They used short multi-walled CNTs (MWCNTs) with lengths of 10-50 µm as the short-range electrical conductive network to support sulfur, as well as super-long CNTs with lengths of 1000-2000 µm from vertically aligned CNTs (VACNTs) as both long-range conductive networks and inter-penetrated binders for the hierarchical free-standing paper electrode. | |
| "We have developed a bottom-up routine in which sulfur was first well dispersed into the MWCNT network to obtain MWCNT@S building blocks and then MWCNT@S and VACNTs were assembled into macro-CNT-S films via the dispersion in ethanol followed by vacuum filtration," Zhe Yuan, a graduate student working with Zhang, and the paper's first author, explains. "These sulfur electrodes with hierarchical CNT scaffolds can accommodate over 5-10 times sulfur compared to conventional electrodes on metal foil current collectors while maintaining the high utilization level of sulfur." | |
| In most reported Li-S cells, aluminum foil was used as current collector and a routine slurry-coating procedure was widely used during fabrication. This resulted in a ratio of 10-50 wt % of binders, conductive agents, as well as modifying precursors in the electrode, which neutralized the advantage of a Li-S system with high specific capacity. | |
| This new fabrication method by the Tsinghua team does not employ aluminum foil or binders. | |
| "With our CNT paper electrodes we were able to achieve an initial discharge capacity of 6.2 mAh cm-2 (995 mAh g-1), a 60 % utilization of sulfur, and a slow cyclic fading rate of 0.20 %/cyc within the initial 150 cycles at a low current density of 0.05 C," Jia-Qi Huang, a co-author of the paper, points out. "The areal capacity can be further increased to 15.1 mAh cm-2 by stacking three CNT-S paper electrodes, with an areal sulfur loading of 17.3 mg cm-2 as the cathode in a Li-S cell." | |
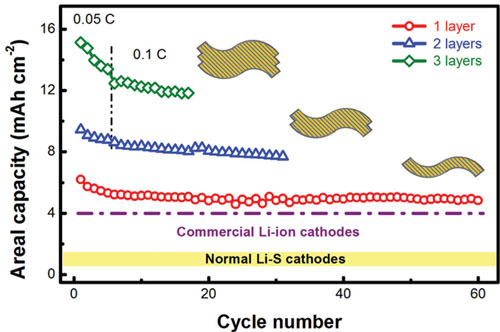 |
|
| High areal capacities of stacked lasagna-like structured electrodes: cycling performance (with insets depicting the corresponding electrode structures). (Reprinted with permission by Wiley-VCH Verlag) | |
| "Our proof-of-concept experiment indicates that the rational design of the nanostructured electrode offers the possibility to efficiently use the active materials at practical loading," says Zhang. "The current bottom-up electrode fabrication procedure is effective for the preparation of large-scale flexible paper electrodes with a good distribution of all functional compounds; this procedure is also potentially applicable to graphene, CNT-graphene, and CNT-(metal oxide)-based flexible electrodes." | |
| This free-standing paper electrode offers the possibility of ubiquitous applications of Li-S batteries at a low cost and at high energy densities for future flexible electronic devices. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
