| Posted: Jul 02, 2015 | |
Pomegranates are good for your renewable energy systems |
|
| (Nanowerk Spotlight) Sustainable energy devices and systems rely on two key electrode reactions: oxygen reduction reaction (ORR) and oxygen evolution reaction (OER). The latter in particular is an enabling process for many energy storage options such as direct-solar and electricity-driven water splitting and rechargeable metal-air batteries. OER suffers from sluggish kinetics – even when facilitated by high activity, precious-metal containing catalysts – and this limitation imposes a significant overpotential requirement. | |
| Researchers have been looking to design catalyst materials that can significantly enhance the performance of OER. Besides the state-of-art but scarce precious metal oxides such as IrO2 and RuO2, transition metal oxides and their derivatives (Ni, Co, Fe, Mn, etc.) are regarded as promising and highly active catalysts for practical OER. | |
| However, common transition metal oxides/hydroxides are poorly conductive and their bulk structure hinders the full accessibility of active sites. Hence, how to finely hybrid metal oxides/hydroxides into a specific conductive substrate to obtain an increased electrochemical active surface area (ECSA), fully exposed active sites, and an optimal interfacial junction is a urgent challenge towards superior OER catalysis. | |
| Recently, carbon nanomaterials such as nanotubes and graphene have been explored as catalyst performance enhancing materials as well (see for instance: "Novel nanocarbon architecture makes a superior bifunctional electrocatalyst"). | |
| "For a heterogeneous electrocatalysis such as OER, both the active phases and conductive supports are key requirements towards superior electrocatalytic activities," Dr. Qiang Zhang, an associate professor at the Department of Chemical Engineering at Tsinghua University, tells Nanowerk. "What is missing is an electrocatalyst with very high electrochemical active surface area, fully exposed active sites, and an optimal interfacial junction." | |
| Zhang is leading a research group that explores and develops advanced materials for efficient electrocatalysis and energy storages. In their most recent work published in the June 26, 2015 online edition of Advanced Materials ("Spatially Confined Hybridization of Nanometer-Sized NiFe Hydroxides into Nitrogen-Doped Graphene Frameworks Leading to Superior Oxygen Evolution Reactivity"), they report the design and fabrication of a novel hybrid of mesoporous graphene and NiFe layered double hydroxides (LDHs) inspired by the hierarchical structure of pomegranate. | |
| "We employed a nitrogen-doped mesoporous graphene framework as the substrate for the in situ growth and decoration of NiFe LDHs," Cheng Tang, the first author explains. "This is the most crucial issue as the nitrogen dopant and topology-induced defects of graphene tend to adsorb and anchor metal precursor and then the in-plane mesopores on graphene serve as nano-reactors for spatially-confined nucleation and growth of NiFe LDHs." | |
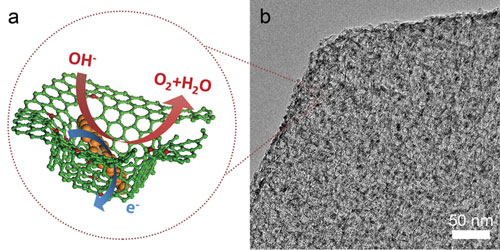 |
|
| a) Schematic of the spatially confined hybrids. Nanometer-sized NiFe LDHs strongly associate with the nitrogen-doped graphene framework, with defect-anchored nucleation and spatially confined growth. Carbon atoms are indicated in green and nitrogen in red. b) Cross-sectional TEM image of a sheet of nanoscale NiFe LDH/NGF electrocatalyst. (Reprinted with permission by Wiley-VCH Verlag) | |
| Just like with pomegranates, where each seed is confined and separated in each sac, in this composite, nano-sized NiFe LDHs are spatially confined in each graphene pore, and also with a strong interfacial couple with the graphene substrate. | |
| "This confined structure decreases the size of LDHs and suppresses the particle aggregation, thereby fully exposing the active sites of LDHs," Zhang points out. "In addition, the intimate interfacial coupling between graphene and LDHs renders an excellent electron transfer. We expected it to perform greatly for OER catalysis." | |
| As the researchers demonstrated, this hybrid system delivered a remarkably low Tafel slope (∼45 mV dec-1), a substantially reduced overpotential (∼337 mV required for 10 mA cm-2), and enhanced durability in 0.10 M KOH. | |
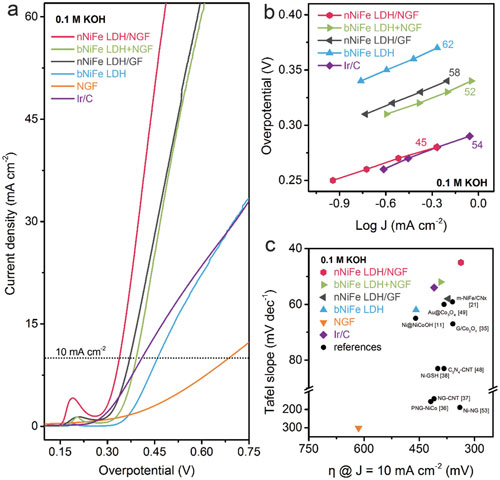 |
|
| a?c) Oxygen evolution catalysis performances of nNiFe LDH/NGF and control samples in 0.1 M KOH electrolyte. a) LSV curves. Scan rate was 5.0 mV s-1. The loading was about 0.25 mg cm-2 for all samples. b) Tafel plots of nNiFe LDH/NGF and other samples for comparison. c) Figures of merit with respect to both kinetics (Tafel slope) and activity (the overpotential required to achieve 10 mA cm-2), with references all measured in 0.1 M KOH electrolyte. (Reprinted with permission by Wiley-VCH Verlag) (click on image to enlarge) | |
| "It overperforms the commercial Ir/C catalysts, and is among the best reported nonprecious metal catalysts," Zhang summarizes the novel material's performance. | |
| "We attribute this superior performance to the synergetic effect of two excellent components and also the unique structure features of this novel hybrid," he adds. "However, further investigations are expected to ascertain the structure-property relationships and optimize the composition and structure of this kind of hybrids." | |
| "We expect this strongly-coupled complex to be inspiring to other researchers and applicable in various fields," concludes Zhang. "But more importantly, the topology-assisted design and fabrication strategy opens up new avenues and sheds light on a novel branch of advanced nano-architectured materials and hybrids." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
