| Posted: Jan 13, 2016 | |
Sulfiphilic electrocatalysts dredge the polysulfide 'flooding' in Li-S batteries |
|
| (Nanowerk Spotlight) Driven by electric vehicles, portable electronics, and storage solutions for sustainable energy harvesting, demand for high-energy-density rechargeable batteries is rising fast. Conventional lithium-ion (Li-ion) batteries, a mature technology that suffers from limited energy density, no longer meet the demanding requirements of these next-generation energy systems. | |
| One promising alternative is the lithium-sulfur (Li-S) battery – employing sulfur as cathode and metallic lithium as anode materials – which theoretically can render 3-6 times higher energy density (2600 Wh kg-1) than lithium-ion batteries. In previous Nanowerk Spotlights we have reported on developments in Li-S research, see for instance "Permselective graphene oxide separator for very stable lithium-sulfur batteries" or "Janus separator: A new opportunity to improve lithium-sulfur batteries". | |
| "The impressively high energy density of a Li-S battery is a result of its unique reaction mechanism," explains Dr. Qiang Zhang, an associate professor in the Department of Chemical Engineering at Tsinghua University in Beijing. "The transformation between sulfur and lithium sulfide involves phase transitions, resulting in a much higher capacity compared to intercalation-type mechanisms. In commonly used aprotic electrolytes, the lithiation of sulfur facilitates a solid-liquid-solid conversion. The soluble intermediates, also known as lithium polysulfides, enhance the redox process and make the high cathode capacity achievable." | |
| Zhang adds that, unfortunately, the soluble polysulfide intermediate is both a blessing and a curse. While contributing to the overall capacity, the solubility of polysulfides is accompanied by their diffusivity, resulting in irreversible loss of active sulfur into electrolyte, anode or dead volumes. | |
| Capacity decay caused by polysulfides’ detachment from the cathode framework has been a major issue preventing the broad application of Li-S batteries. | |
| "To date, the commonly used strategy to resolve this problem has been to suppress polysulfide diffusion by adopting functional interlayers, anode-protecting additives, and novel electrolyte configurations," Zhe Yuan, the first author of a paper on this work, comments on the status quo. "Nonetheless, the overflow of polysulfides in the electrolyte should not be only imputed to their inevitable diffusion. The slow redox reaction rate of polysulfide intermediates is also to blame." | |
| Zhang and his team got inspired by an intriguing analogy of polysulfide overflow – an actual flood. | |
| "Compared to blocking the deluge with embankments, digging and enlarging canals or channels are seemingly more effective approaches in mitigating flooding," says Qiang. "Similarly, expediting polysulfide redox reaction, which is originally sluggish, removes the barrier of polysulfide consumption, and therefore lessens the detrimental effects induced by polysulfide accumulation in the electrolyte." | |
| The researchers discovered that it was the incompatibility between polar lithium polysulfide molecules and commonly used nanocarbon cathode scaffolds that restrained the redox reactivity. Nanocarbon materials are excellent for Li-S batteries since they are highly conductive and porous. But their non-polar surface characteristic do not lend themselves favorably to being attached to heteropolar polysulfides. | |
| "In that sense, we speculated that it would be advantageous to add a polar substance with high affinity with polysulfides into the cathode framework, and it turned out to be true," notes Zhe. | |
| This beneficial additive is cobalt disulfide (CoS2), a half-metallic earth-abundant mineral. The team imported CoS2 into graphene frameworks by facile mechanical mixing. The modified cathodes armed with enhanced interactions between CoS2 and lithium polysulfides exhibited substantially propelled polysulfide redox reactions, promoted energy efficiencies and elevated discharge capacities, as reported in the December 29, 2015 online edition of Nano Letters ("Powering Lithium–Sulfur Battery Performance by Propelling Polysulfide Redox at Sulfiphilic Hosts"). | |
| "CoS2 has appreciable room temperature conductivity, and more importantly, it possesses a sulfiphilic surface," says Ting-Zheng Hou, a graduate student in Qiang's group and a co-first author of the paper. "The latter feature guarantees smooth attachment of polysulfides onto CoS2 surfaces, and the former one provides efficient electron pathways to facilitate redox reactions. The incorporation of CoS2 significantly enhances the electrochemical reactivity of soluble lithium polysulfides." | |
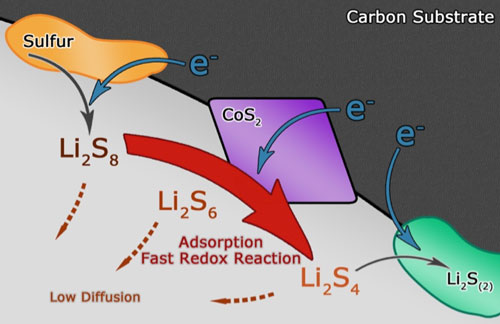 |
|
| Schematic illustration of the discharge process in CoS2-incorporated carbon/sulfur cathode, where polysulfide reduction is accelerated and polysulfide diffusion is weakened. (Image courtesy of the researchers) | |
| The Tsinghua team demonstrated that a 62% capacity boost and a 10% energy efficiency increase at a current density of 0.5 C could be achieved simply by adding 15 wt% CoS2 into a graphene framework, which could be attributed to the accelerated polysulfide redox kinetics. | |
| "The composite cathode also exhibited excellent stability, giving a low capacity decay rate of 0.034% percycle in 2,000 cycles. These results are consistent with our hypothesis," says Hong-Jie Peng, another co-first author of the paper. "Moreover, the electrocatalytic effect of CoS2 on polysulfide redox, namely the liquid-liquid conversion, in Li-S batteries was investigated through symmetric cells for the first time." | |
| Qiang points out that it may seem counter-intuitive that the liquid-liquid conversion between polysulfide species is the kinetically slowest step in the S-polysulfides-Li2S sequence. | |
| "However" he says, "electrochemical reactions are heterogeneous and heavily dependent on the chemisorption behavior of the reactant. The addition of CoS2 fundamentally alters the dynamic pattern of sulfur distribution among all species, resulting in differentiated morphology at different charging or discharging states." | |
| "Generally speaking" he concludes, "CoS2 serves as an electrocatalyst and opens up a new wide canal for polysulfides to be consumed instead of being accumulated." | |
| Since their dredging strategy turned out to be effective, Zhang and his colleagues are planning to further explore novel cathode designs based on the modulation of the redox chemistry of Li-S batteries and beyond. | |
| "Delicate compositions of nanocarbon and inorganic materials could be applicable to a vast number of energy storage and conversion devices," says Qiang. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
