| Posted: Oct 04, 2016 | |
New study shows that iron oxide nanoparticles inhibit tumour growth |
|
| (Nanowerk Spotlight) The intravenous iron-replacement product ferumoxytol and other iron oxide nanoparticles are being used for treating iron deficiency, as contrast agents for magnetic resonance imaging, and as drug carriers. | |
| In a new study, for the first time, researchers have shown an intrinsic therapeutic effect of ferumoxytol on the growth of early mammary cancers and lung cancer metastases in liver and lungs. They showed that ferumoxytol can activate the immune system to attack cancer cells. | |
| "This is the first description of an intrinsic therapeutic effect of iron oxide nanoparticles against cancer," Saeid Zanganeh, PhD, a postdoctoral scholar in the Department of Radiology, Molecular Imaging Program at Stanford University, and first author of a paper on this work (Nature Nanotechnology, "Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues"), tells Nanowerk. | |
| "Our data have broad implications for diagnostic and therapeutic nanoparticle applications," says Morteza Mahmoudi, an assistant professor at Tehran University of Medical Sciences, who heads the Laboratory of Nano-Bio Interactions, and co-author of the paper. "Since ferumoxytol is FDA-approved for intravenous treatment of iron deficiency, it could be applied 'off label' to protect the liver in patients from metastatic seeds and potentiate TAMs-modulating cancer immunotherapies." | |
 |
|
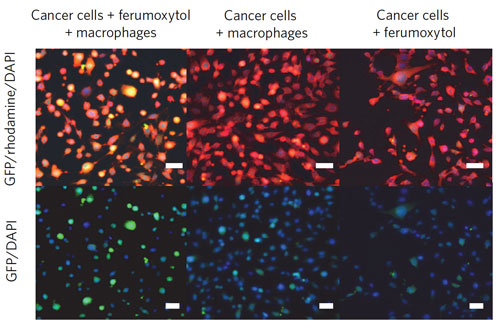
| Combining ferumoxytol and macrophages leads to cancer cell apoptosis through the Fenton reaction. RAW264.7 macrophages were co-cultured with MMTV-PyMT cancer cells in a transwell system for 24 h, with or without ferumoxytol (2.73 mg ml–1). Macrophages in the upper chamber and cancer cells in the lower chamber were separated by a 0.4-µm-sized microporous membrane, which allowed for exchange of molecules, but not cells. Cancer cells in the lower chamber were stained for caspase expression using green fluorescent protein (GFP)-conjugated antibodies against active caspase 3 (green). F-actin and the cell nuclei were counterstained with phalloidin rodamine (red) and DAPI (blue), respectively. Images show that co-culture of cancer cells, macrophages and ferumoxytol leads to increased caspase-3 expression of cancer cells. Co-incubations of cancer cells and macrophages only or cancer cells and ferumoxytol only do not lead to significant apoptosis induction. Scale bars, 20 µm. (© Nature Publishing Group) (click on image to enlarge) | |
| Previous studies have described the use of iron oxide nanoparticles for targeted delivery of therapeutics (see for instance our previous Nanowerk Spotlight: "Smart cancer nanotheranostics"). In these studies, the nanoparticles served as carriers of therapeutic drugs to the tumor microenvironment. | |
| Conversely, in this new study, the scientists observed an intrinsic therapeutic effect of iron oxide nanoparticles themselves. | |
| "Our proposed approach to suppress liver metastases by repetitive systemic ferumoxytol administrations should be achievable without significant impact on iron load or liver toxicity," notes Zanganeh. | |
| He adds that, due to the large surface area and chemically defined surface structure, iron oxide nanoparticles such as ferumoxytol can be conjugated or functionalized to numerous ancillary molecules to further amplify the desired immune-modulating properties. | |
| The scientists explain that ferumoxytol could be locally delivered to unresectable tumors via interventional procedures, to micrometastases in confined spaces – e.g. peritoneal seeds. Or to tumor resection margins at the end of a tumor surgery with positive resection margins, in order to induce a pro-inflammatory reaction against cancer cells and suppress tumor growth in the interval between a tumor surgery and initiation of postsurgical chemotherapy or irradiation. | |
| On the other hand, patients with primary tumors that typically metastasize to the liver could receive protective ferumoxytol medications to prevent early metastatic seeds. | |
| In the next stage of their study, the researchers will be more focused on the evaluations of nanoparticle-drug combinations. Potential clinical applications of ferumoxytol-mediated pro-inflammatory immune responses could entail potentiating the efficacy of other M1-activating cancer immunotherapies, such as anti-CD47 mAbs or antibodies blocking IL-4 or IL-13 signaling. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
