| Posted: Mar 11, 2008 | |
Understanding the interactions of nanostructures with biological systems |
|
| (Nanowerk Spotlight) In the good old days of nanotechnology, some two to three years ago, it became clear that researchers were making fascinating progress in revolutionizing drug delivery by using nanoparticles as delivery agents. Previously, the ideal drug carrier was something out of science fiction: when injected into the body it transports itself to the correct target, such as a tumor, and delivers the required dose at this target. With the advent of nanomedicine, this idea, nicknamed the 'magic bullet' concept, is rapidly becoming a reality. Currently used pharmaceutical nanocarriers like liposomes, micelles, nanoemulsions, polymeric nanoparticles and many others demonstrate a broad variety of useful properties, such as for instance increased longevity in the blood, specific targeting to certain disease sites, or enhanced intracellular penetration. Researchers have also begun to combine several properties in order to develop multifunctional nanocarriers. Overall, these nanocarriers already have proven quite successful in practice. | |
| However, as scientists begin to understand more and more of the intricate issues involved in designing nanoparticulate drug carriers, and actually begin to use them in real-life scenarios and clinical tests, they realize that the design parameters for their therapeutic wunderkinder are more critical than they initially realized. Just a few days ago we had Dr. Mauro Ferrari tell us that his group's research results indicate that almost all the nanocarriers that are in the clinic or in the preclinical pipeline are basically the worst possible size and shape for their intended purpose. | |
| And now, a new study in Canada reveals that nanoparticles do not just act as simple, passive carriers but are actively involved in mediating biological activity. These findings have significant implications in understanding the interactions of nanostructures with biological systems. But, once properly understood, they could be important in assisting in the design of intelligent nanodevices, with great potential for the development of novel molecular-based diagnostics and therapeutics. On the other hand, they could also be useful in understanding nanotoxicity. | |
| In spite of what has been achieved so far by scientists and clinical researchers, a complete understanding of how cells interact with nanostructures of well-defined sizes, at the molecular level, remains poorly understood. Dr. Warren Chan, an Assistant Professor at the University of Toronto's Institute of Biomaterials and Biomedical Engineering, together with students Wen Jiang and Dr. Betty Kim and collaborator Dr. James Rutka at the The Hospital for Sick Children, show that gold and silver nanoparticles coated with antibodies can regulate the process of membrane receptor internalization. | |
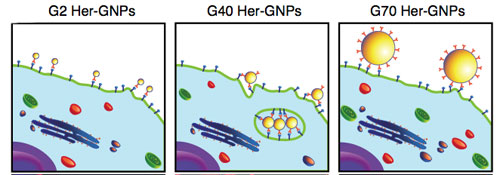
| The scientists found that the binding and activation of membrane receptors and subsequent protein expression strongly depend on nanoparticle size. Although all nanoparticles within the 2–100 nm size range were found to alter signaling processes essential for basic cell functions – including cell death – 40- and 50-nm nanoparticles demonstrated the greatest effect. | |
| "We have demonstrated that engineered nanoparticles of well-defined sizes can selectively induce membrane receptor internalization to downregulate their expression level" Chan tells Nanowerk. "This in turn alters the downstream signaling and subsequent cellular responses. Our findings provide strong evidence that nanostructures can not only passively interact with cells, but also actively engage and mediate the molecular processes that are essential for regulating cell functions." | |
 |
|
| Dependence of downregulation of membrane ErbB2 expression on nanoparticle size. Illustrations with corresponding fluorescence images of ErbB2 receptor localization after treatment with different-sized Her-GNPs. Arrows indicate ErbB2 receptors, and the nucleus is counterstained with DAPI (blue) (scale bars=10µm). (Image: Dr. Chan, University of Toronto) | |
| The researchers reported their findings in the March 2, 2008 online edition of Nature Nanotechnology (Nanoparticle-mediated cellular response is size-dependent) | |
| "What these results tell us is that nanoscientists need to carefully purify and analyze nanoparticles before they use them in biomedical applications – otherwise the particle may not elicit the desire biological response," says Rutka. | |
| The understanding of how engineered nanoparticles of different geometries interact with cells requires the study of the molecular events involved in nanoparticle-membrane receptor binding, endocytosis and subsequent signaling activation. Chan says that the characterization of these molecular processes, which can provide novel means to modulate cellular behaviors, has not been explored. | |
| Previous studies showed that uptake kinetics are dependent on size and shape of nanoparticles. Chan's study takes it a step further and shows the molecular effect of different sizes of a nanoparticle after it interacts with a cell. | |
| "To demonstrate that engineered nanoparticles of well-defined sizes actively participate in the processes of regulating and modulating cellular responses, we synthesized multivalent engineered gold nanoparticles to selectively control specific interactions between Herceptin (an antibody used in anti-cancer therapy in breast cancer) and its receptor ErbB2" Jiang, the paper's first author, explains the study. "The attachment of multiple Herceptin molecules onto the nanoparticle surface allows the formation of multivalent engineered nanoparticles for crosslinking surface ErbB2 receptors and to alter cell fate." | |
| To analyze the size-dependent internalization of the Herceptin-functionalized gold nanoparticles (Her-GNP), Chan's team studied the binding dynamics between Her–GNPs and ErbB2 receptors. | |
| Since the number of allowable Herceptin binding sites on nanoparticles is dependent on the particles' surface area, this number increases if particle size increases. In addition, antibody density on the particle surface increases linearly with respect to its radius owing to the reduction in the average area covered by each protein. | |
| "The higher surface curvature of smaller nanoparticles restricts the relative orientation between molecules with a certain degree of conformational rigidity and their docking surface during the adsorption process, resulting in large background areas without protein coverage" says Kim, one of the co-authors. "Larger nanoparticles have a higher protein-to-nanoparticle ratio, which allows the restructuring of protein postures on the nanoparticle surface to maximize protein loading. The overall increase in protein adsorption correlates with particle size, which significantly enhances the multivalency of Her–GNPs." | |
| These findings allowed the UToronto scientists to conclude that their multivalent antibody nanoparticles exhibit a high degree of ErbB2 crosslinking capability, which is tunable by nanoparticle size. | |
| As a next step, Chan and his team will attempt to validate the therapeutic effects and size-dependence of Herceptin-coated nanoparticles in animal studies. | |
| Chan cautions though that the major challenge for their research is the speed of analysis. "These kind of studies are fundamental to the field of nanomedicine, and nanotechnology in general, but they take a lot of time to do." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
