| Posted: Mar 06, 2018 | |
Contact lenses with built-in biofuel cells as power supply |
|
| (Nanowerk Spotlight) Enzymatic biofuel cells (EBFCs) are bioelectronic devices that utilize enzymes as the electrocatalysts to catalyze the oxidation of fuel and/or the reduction of oxygen or peroxide for energy conversion to electricity. Compared to traditional fuel cells that use conductive-metal nanoparticles as catalysts, these fuel cells work with living organisms that can consume and metabolize complex fuels such as sucrose, fructose, etc. | |
| EBFCs have been demonstrated as wearable epidermal tattoo biosensors to harvest energy from lactate present in human sweat during physical exercise (for more details see: Electroanalysis, "Tattoo-Based Wearable Electrochemical Devices: A Review"). | |
| Already in 2012, researchers demonstrated a biofuel cell as a power source for electronic contact lenses. | |
| In new work (ACS Applied Materials & Interfaces, "Nanoporous Gold-Based Biofuel Cells on Contact Lenses"), researchers report the fabrication of flexible nanoporous gold electrodes that were modified with lactate oxidase and bilirubin oxidase for use as a lactate/O2 biofuel cell. | |
| This flexible EBFC holds potential as an autonomous power supply for wearable electronic devices. | |
 |
|
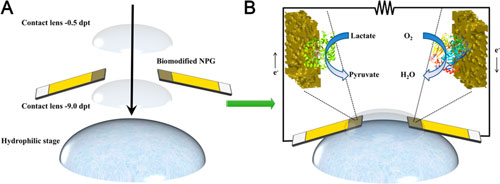
| Schematic diagram of the assembly of the A) modified contact lens and B) the configuration of the EBFC. (Reprinted with permission by American Chemical Society) (click on image to enlarge) | |
| The team placed the electrodes between two commercially available contact lenses to avoid direct contact with the eye and tested them in a solution of artificial tears as well as one containing phosphate buffer solution. Hydrophilic silicon-hydrogel contact lenses contain microchannels that enable the transport of solutions and oxygen to the EBFC. | |
| The EBFC contact lenses achieved a maximum power density of 1.7 ± 0.1 µW cm-2 and an open-circuit voltage of 380 ± 28 mV, values slightly lower than those obtained in phosphate buffer solution (2.4 ± 0.2 µW cm-2 and 455 ± 21 mV, respectively). | |
| Ascorbate interference, especially at the biocathode, was responsible for the decrease in performance in tears in comparison to the performance in phosphate buffer solution. A coating film on the biocathode may alleviate such interference effects. | |
| The decrease was mainly attributed to interference from ascorbate. After 5.5 h of operation, the EBFC retained 20% of the initial power output. | |
| The authors note that the response of the assembled EBFC was limited by current density and operational stability of the biocathode. Improvements in the observed current density of the biocathode will enable the development of a self-powered lactate biosensor on a contact lens, where the power density of the EBFC could be correlated with the concentration of lactate. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
