| Oct 09, 2018 | |
Beware the fake graphene |
|
| (Nanowerk Spotlight) Peter Bøggild over at DTU just published an interesting opinion piece in Nature titled "The war on fake graphene". | |
| The piece refers to a paper published in Advanced Materials ("The Worldwide Graphene Flake Production") that studied graphene purchased from 60 producers around the world. | |
| This study used a systematic and reliable protocol that the authors developed to test graphene quality using optical microscopy (to identify flake size); atomic force microscopy (to measure the thickness of graphene stacks); Raman spectroscopy (which provides information on the structural integrity of the sample as well as indicates the presence of graphene oxide and reduced graphene oxide); X-ray photoelectron spectroscopy (to measure the carbon content to establish purity); and scanning electron microscopy and transmission electron microscopy to further characterize sample morphology. | |
| The study's findings show unequivocally "that the quality of the graphene produced in the world today is rather poor, not optimal for most applications, and most companies are producing graphite microplatelets. This is possibly the main reason for the slow development of graphene applications, which usually require a customized solution in terms of graphene properties." | |
| A conclusion that sounds even more damming is that "our extensive studies of graphene production worldwide indicate that there is almost no high quality graphene, as defined by ISO, in the market yet." | |
| The team also points out that a large number of the samples on the market labelled as graphene are actually graphene oxide and reduced graphene oxide. Furthermore, carbon content analysis shows that in many cases there is substantial contamination of the samples and a large number of companies produce material a with low carbon content. Contamination has many possible sources but most likely, it arises from the chemicals used in the processes. | |
| Contamination also affects the number of sp2 bonds. The study found that "crystalline graphene should have 100% sp2 bonds. However, we were not able to find, in any of the companies studied, a sample with more than 60% sp2 bonds." | |
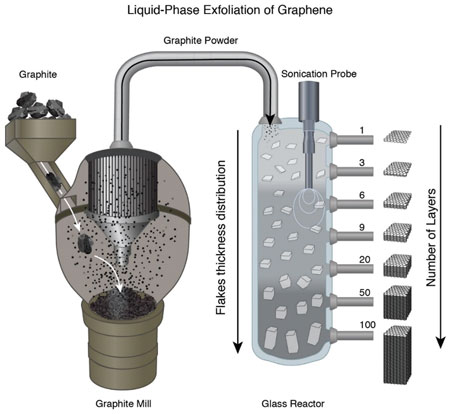
| Among the established methods for commercial graphene production, liquid-phase exfoliation (LPE) of graphite is one of the methods used most frequently. The mechanism behind LPE is based on the fact that graphite is a layered material and essentially can be seen as individual graphene crystals stacked one on top of each other. | |
| The LPE process involves milling graphite into a powder, and separating the particles into tiny flakes by applying mechanical forces in a liquid. The graphene-containing flakes are then separated from the remaining material. | |
 |
|
| Liquid-phase exfoliation schematic process. In the chemical reactor of LPE the lighter products such as stacks with very few layers float to the top of the solution while the heavier products such as graphite remain at the bottom of the reactor (there are many practical ways how to achieve it, including gravimetric, centrifugation, etc.). It is possible to extract each product and repeat the process several times to get higher concentration of monolayers but this, of course, impacts on cost. (© Nature) | |
| The authors argue that the creation of stringent standards for graphene characterization and production, taking into account both the physical properties, as well as the requirements from the particular application, is the only way forward to create a healthy and reliable worldwide graphene market. | |
| Driving home the point that careful characterization is key to the developing market for graphene products, the authors mention that different applications would require different grade of graphene: "For instance, the best performance in composite materials applications would be achieved with large flakes which are two to three monolayer thick. At the same time, the best electrodes for neurological applications are prepared from monolayer flakes, etc. We envisage that each particular application would require fine-tuning of the properties of the graphene material (in terms of its thickness and size distributions, basal plane and edge functionalization, etc.)." | |
| In his opinion piece, Bøggild cautions that it should be noted that the study does not cover all the types of bulk graphene on the market. "Moreover, although the authors analyses an impressive number of LPE-manufactured products, they could have eliminated any accusations of potential bias by specifying the criteria they used to select the products for analysis." | |
| He continues that it is also possible that they unintentionally missed high-quality graphene sold by a few excellent producers. And, as the researchers mention, different applications generally make use of different characteristics of graphene – which makes it difficult to come up with a universal metric of quality. | |
| "Nevertheless" he concludes, "the work is a timely and ambitious example of the rigorous mindset needed to make rapid progress, not just in graphene research, but in work on any nanomaterial entering the market. To put it bluntly, there can be no quality without quality control." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
