| May 06, 2019 | |
Building large graphene aerogel walls |
|
| (Nanowerk Spotlight) Graphene is a single layer of carbon atoms organized in a hexagonal lattice. When graphene sheets are neatly stacked on top of each other and formed into a three-dimensional shape, it becomes graphite, commonly known as the lead in pencils. | |
| Because graphite is simply packed-together graphene, it has fairly poor mechanical properties. But if the graphene sheets are separated with air-filled pores, the three-dimensional structure can maintain its properties. This porous graphene monolith structure is called a graphene aerogel. When the gas phase is replaced by a liquid, without the graphene network losing its stability, then we speak of a hydrogel. | |
| Highly compressible graphene aerogels possess extraordinary properties that exceed the performance of natural materials – superior compressive elasticity; ultrahigh porosity; outstanding tolerance for harsh environment; large specific surface area; high electrical and thermal conductivity. | |
| These aerogels have shown great potential as multifunctional frameworks to be applied in such areas as high-performance damping, large strain sensors, fast oil-water separation, flame retardation, thermal insulation, electromagnetic interference shielding, and sound absorption. | |
| However, very low mechanical strength and limited macroscale have hampered the practical applications of graphene aerogels. | |
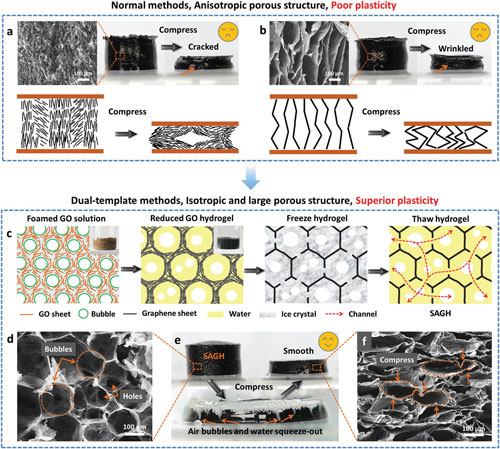
| A team of researchers from Beijing Institute of Technology and Tsinghua University have developed a novel sol-gel method by introducing air bubbles and ice crystals as dual templates to prepare graphene hydrogels that can be air dried without shrinkage. | |
| The air bubbles are introduced in the graphene oxide solutions as the soft template to produce the isotropic large pores in the reduced graphene hydrogels. The ice crystals impale the graphene walls to introduce many interconnected channels among the isotropic large pores. | |
 |
|
| Compression process of graphene hydrogels with anisotropic porous structure and poor plastic deformation prepared by normal methods, such as a) ascorbic acid reduction of 4 mg mL-1 GO solution and further b) freeze-thaw process at -10°C. c) Schematics illustration of the developed dual-template (air bubble and ice crystal) sol–gel method for preparing superelastic air-dryable graphene hydrogel (SAGH) with isotropic, open-cell, and large porous structure. d) SEM image of the cross-section of a typical SAGH. e) Compression process of SAGH with air bubbles being squeezed out and superior plastic deformation. f) SEM image of the cross-section of SAGH after compression. (Reprinted with permission by Wiley-VCH Verlag) (click on image to enlarge) | |
| Reporting their findings in Advanced Functional Materials ("Superplastic Air-Dryable Graphene Hydrogels for Wet-Press Assembly of Ultrastrong Superelastic Aerogels with Infinite Macroscale"), the team employs a facile wet-press assembly strategy to fabricate ultrastrong, superelastic graphene aerogels with infinite macroscale. | |
| After the graphene hydrogel is prepared by the team's sol-gel method, it is compressed in order to squeeze out the air bubbles and solution through the interconnected channels in the gel. The remaining structure is then dried in air to enhance the stiffness of the 3D framework. | |
| "The dried aerogel doesn't show any obvious volume shrinkage, a result that we attribute to the framework stiffness of the gels, which could resist the solvent evaporation capillary pressure," Professor Liangti Qu from the Beijing Institute of Technology, who led this work, tells Nanowerk. "An unlimited number of these graphene aerogel 'bricks' can be assembled together during the pressing process, much like building a wall with LEGO® bricks." | |
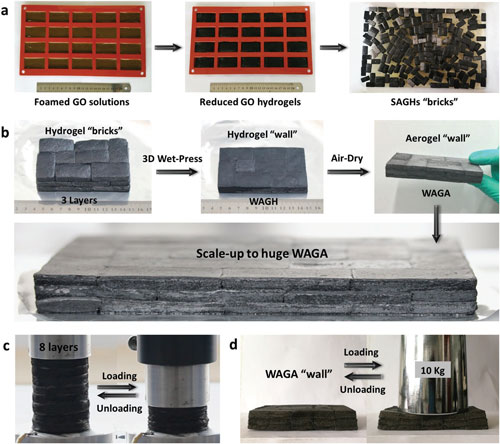
| The researchers demonstrated that the combined graphene aerogels form a highly oriented, dense, multiple-arch microstructure that possesses arbitrary macroscale, outstanding compressive strength (47 MPa, over 10 times higher than the best ever reported), superelasticity (>97% strain), and high conductivity (378 S m-1). | |
 |
|
| a) Large-scale preparation of SAGH “bricks” with foamed GO solutions being poured into the cuboid moulds. b) Wet-press assembly process of huge WAGA “wall” prepared by SAGH “bricks” after air-drying. c) Uniaxial compression and recovery process of the eight-layer WAGA at 70% compressive strain. d) Loading and unloading process of the WAGA “wall” by a 10 kg weight. (Reprinted with permission by Wiley-VCH Verlag) (click on image to enlarge) | |
| Qu notes that this ultrastrong, superplastic graphene aerogel could find applications as advanced multifunctional structural materials for buildings, spacecraft, and vehicles. | |
| "Our next steps will include investigations with regard to extending their fabrication strategy to other graphene-based hybrid and composite aerogels and improving and expanding the functionality of the aerogels," he says. "We also would like to develop more facile and energy-efficient fabrication methods in order to make this process attractive for commercialization." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
