| Oct 31, 2019 | |
The rise of graphynes |
|
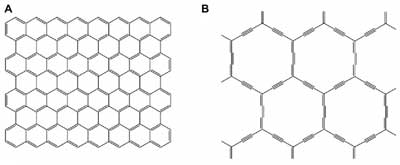
| (Nanowerk Spotlight) Graphyne is a little known sibling of graphene. Simulations have shown that graphyne's conduction electrons travel extremely fast – as they do in graphene – but in only one direction. That property could help researchers design faster transistors and other electronic components that process one-way current. | |
| Graphyne is distinct in being composed of sp and sp2 carbon atoms, which contrasts with graphene containing only sp2 carbon. | |
 |
|
| Figure 1. Structures of A) graphene and B) α-graphyne. (Reprinted with permission by The American Physical Society). | |
| The term graphyne was first used by Baughman and co-workers in 1987 (The Journal of Chemical Physics, "Structure-property predictions for new planar forms of carbon: Layered phases containing sp2 and sp atoms"), who estimated its structure, thermodynamics, and electronic properties. | |
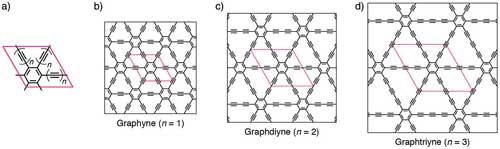
| Graphynes and graphdiynes are generic names for families of 2D carbon allotropes with honeycomb structure, where acetylenic groups connect the hexagons of graphene, with the coexistence of sp and sp2 hybridized carbon atoms. The main differences between graphynes and graphdiynes are the number of acetylenic groups (one for graphynes and two for graphdiynes). | |
 |
|
| Figure 2. Chemical structures of key members of the graphyne family. a) Chemical structure of the major types in the graphyne family. b–d) 2D lattices of graphyne, graphdiyne, and graphtriyne. (Reprinted with permission by Wiley-VCH Verlag) (click on image to enlarge) | |
| To date, the only graphyne to have been synthesized is graphdiyne (which corresponds to n=2 in Figure 2), which was made in 2010 (Chemical Communications, "Architecture of graphdiyne nanoscale films"). | |
| Among the graphyne family, graphdiyne also has received the most theoretical attention. Calculations have shown it to be a semiconductor with a bandgap of 0.5–1.3 eV. The bandgap can be varied continuously to a zero-gap metal or a semimetal via lamination and transformation into nanoribbons or nanotubes. | |
| In addition, graphdiyne is expected to have a carrier mobility at room temperature greater than that of silicon, and rivaling that of graphene owing to properties such as its small effective mass. | |
| A recent paper by Japanese scientists in Advanced Materials ("The Accelerating World of Graphdiynes") comprehensively reviews the literature on graphynes, and specifically graphdiynes. | |
| In it, they describes the history and structure of graphynes; summarize analogs that have been reported based on the molecule-based nanosheet nature of graphdiyne; examine the weaving of 2D lattices of graphynes from their molecular monomer components; survey theoretical and experimental studies of graphdiyne and its physical properties; and discuss the state-of-the-art research on the applications of graphynes as new carbon materials. | |
 |
|
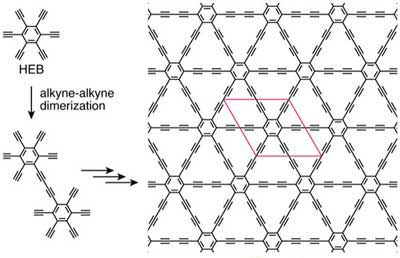
| Figure 3. Synthetic scheme and chemical structure of graphdiyne (HEB = hexaethynylbenzene). (Reprinted with permission by Wiley-VCH Verlag) | |
| Graphdiyne not only is a 2D carbon allotrope but also a molecular covalent organic nanosheet (CON) because it can be synthesized from an organic monomer, HEB, via controlled oxidative homocoupling. The bottom-up approach can provide a variety of graphyne derivatives by tuning the organic monomer. | |
| The coexistence of sp and sp2 carbons in graphyne gives rise to unique physical properties, such as high conductivity and large carrier mobility. This makes it a promising candidate for several application areas (which the authors discuss in great detail in their review): electrocatalysis; organic reaction catalysis; energy storage devices; photocatalysts and solar cells; water purification; and sensors. | |
| "Research on the graphyne family has begun in earnest, and further work will ensure it fulfils its potential," the authors conclude. "As a closing remark, we see it approaching its zenith in the forthcoming decade and shining light on various interdisciplinary fields." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
