| Oct 08, 2020 | |
Sperm-powered micro trucks |
|
| (Nanowerk Spotlight) Bio-hybrid micromotors are devices equipped with specific propulsion mechanisms such as local chemical reactions, remote acting forces, or motile bio organisms, that move on a small scale and are a combination of biological and artificial components. | |
| Especially biomedical micromotors are attracting much attention due to their controllable motion, their minimal invasiveness, and their versatility for diverse biochemical functions. On the way to the next generation of medical treatments, these tiny devices are expected to perform various operations with high precision such as targeted drug delivery, microsurgery, and sensing for diagnostic purposes. | |
| Researchers are already experimenting with different designs and fuel systems for micromotors that can travel in water, blood and other body fluids. Back in 2015, we reported on the first demonstration of micromotor operation and payload release in a living organism. | |
| A previous Nanowerk Spotlight ("Spermbots – microrobotics meets sperm cells") summarized the advances in the integration of single sperm cells in microrobotic devices and illustrates how wide-ranging the potential of these reproductive cells is. | |
| Recent work by a team of researchers at the Institute for Integrative Nanosciences, Leibniz IFW Dresden, published in ACS Nano ("Sperm Micromotors for Cargo Delivery through Flowing Blood") presents streamlined-horned cap (SHC) hybrid sperm micromotors, which can efficiently and controllably swim against flowing blood. | |
| As the authors note, their study not only initiates application scenarios for micromotors in the circulatory system but also envisions an administration route of sperm or sperm-hybrid micromotors for future local medical applications. | |
| Sperm micromotors swimming in 10× diluted blood in a 1 mm thick microchannel. | |
| Different from other in vivo applications, any medical mission in blood faces a particularly complex set of challenges for researchers; this includes the presence of various substances and cells, complex hemorheological properties, and high flow. | |
| Sperm-based micromotors hold several advantages, in particular their powerful propulsion generated by the beating of the sperm flagella. Sperm are also able to swim for several days with a drag force up to 100 pN, which is comparable to the propulsion force reported by some of the most powerful catalytic micromotors (ranging from 3.77 to 500 pN). | |
| The most important feature of sperm micromotors to operate in the bloodstream, the team points out, is their ability to swim against flow (rheotaxis) as well as close to boundaries (thigmotaxis), where the blood velocity is lower than the average velocity, due to shear stress. These two guidance mechanisms are the most efficient ones for guiding mammal sperm towards the fertilization site. | |
| In combination with a synthetic magnetic scaffold (cap), sperm micromotors can be precisely guided and operated by an external magnetic field to further release a cargo at the right place and time by making use of cutting-edge imaging techniques. | |
 |
|
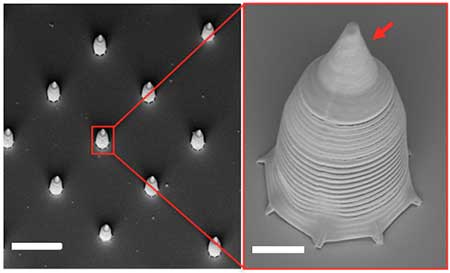
| SEM images of a horned cap. The red arrow indicates the horn structure used for enhancing the micromotor motion in blood. Scale bars: 40 µm in the left panel and 5 µm in the right panel with higher magnification. (Reprinted with permission by American Chemical Society) | |
| The design of the team's sperm micromotor consists of a horn-like, tubular cap with a diameter of ca. 15 µm that was fabricated by two-photon lithography. The horn-like structure enhances the micromotor's motion in blood. For single sperm micromotors, the microcaps were coated with 2 nm of Ti, 15 nm of Fe, another 2 nm of Ti, and 10 nm of SiO2. | |
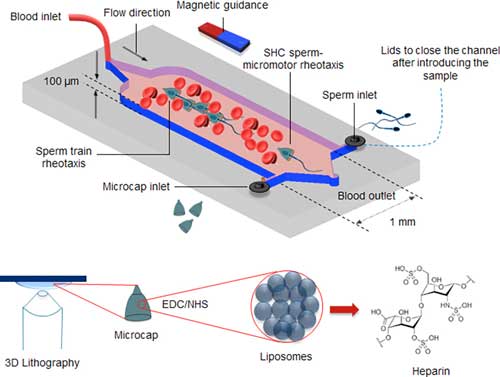
| As a proof-of-principle of a potential medical use, the scientists functionalized the microcaps with heparin-loaded liposomes to realize a localized anticoagulant function. They then mixed the microcaps with sperm, which they added into 4x diluted blood. When free-swimming sperm get caught inside the cap they then propel this cap. | |
| The authors report that the functionalized sperm micromotors showed a significant anticoagulant effect, whereas the nonfunctionalized sperm micromotors did not. In the future, they plan to equip the immobilized liposomes with thermosensitive shells or functional groups that activate certain coagulation factors toward triggered release or specific targeting. | |
 |
|
| Concept of the blood-adapted SHC sperm micromotors. (Reprinted with permission by American Chemical Society) (click on image to enlarge) | |
| As a step beyond single sperm micromotor guidance, the team employed a different coating strategy to induce the self-assembly of multiple sperm micromotors into a train-like configuration. This was achieved by depositing Fe at a 75° angle. When a uniform magnetic field is applied across the sample with randomly dispersed SHCs and sperm, the magnetic layer on each SHC acquires a dipole configuration, leading to the alignment of SHCs along the direction of the magnetic field. | |
| This video shows the guided locomotion of the sperm train in the sperm medium. During the guidance, the sperm train is still capable of assembling with other sperm trains, breeding a new longer sperm train but with a lower swimming speed. Finally, when the sperm train arrives at the desired position, we can easily trigger the coupled sperm to escape by repeatedly flipping the SHCs and dissembling the sperm train by abruptly turning the magnetic field. | |
| The authors conclude that their results not only exhibit a successful blood-adapted sperm micromotor but also serve to identify the minimum requirements that any other medical microrobot should fulfill to be able to operate in blood vessels. "Although we are aware that additional optimizations might be necessary before moving to animal trials, we believe this is the first micromotor that has satisfied most of the key needs to safely and efficiently operate in flowing blood due to some noteworthy advantages: strong sperm propulsion, sperm rheotaxis, biocompatibility, and the capability of loading therapeutics in both the sperm and microcaps." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
